16.4:
Calculating pH Changes in a Buffer Solution
A slight pH change occurs in a buffer solution upon the addition of a small amount of strong acid or the addition of a small amount of strong base.
This pH change is calculated in two distinct steps.
First, a stoichiometric calculation is used to determine the change in the concentrations.
Then, an equilibrium calculation is used to determine the new pH of the solution, either using an ICE table or the Henderson-Hasselbalch equation.
The pH of a buffer containing 2.0 M of both hydrofluoric acid and sodium fluoride can be calculated before and after adding a strong acid or base.
The Henderson-Hasselbalch equation can be used to determine the initial pH of this buffer because the concentrations are high relative to the Ka of the weak acid, and the change in the concentrations is less than 5%.
The pKa for hydrofluoric acid can be calculated to be 3.46. When the concentration of weak acid and conjugate base in a solution are equal, the pH is equal to the pKa. Therefore, the initial pH is 3.46.
If 0.2 moles of hydrochloric acid is added to one liter of this buffer, with the assumption that it causes a negligible change in volume, the added acid is neutralized by the fluoride ions, producing hydrofluoric acid. This leads to a stoichiometric decrease in the concentration of fluoride ions by 0.2 moles and an equal increase in hydrofluoric acid concentration.
The new concentrations can be inserted into the Henderson-Hasselbalch equation. When solved, the new pH is 3.37, less than the initial pH value of 3.46.
As strong acid was added, there is a decrease in the pH, but the reduction is small because the solution is buffered.
In contrast, if 0.1 moles of sodium hydroxide is added to one liter of the buffer, with the assumption that it causes a negligible change in volume, the added base is neutralized by reacting with hydrofluoric acid. This causes a stoichiometric decrease in hydrofluoric acid concentration by 0.1 moles and an equal increase in fluoride ion.
Using the Henderson-Hasselbalch equation, the pH of the solution is 3.50, which is slightly higher than the initial pH value of 3.46 due to the addition of sodium hydroxide.
16.4:
Calculating pH Changes in a Buffer Solution
A buffer can prevent a sudden drop or increase in the pH of a solution after the addition of a strong acid or base up to its buffering capacity; however, such addition of a strong acid or base does result in the slight pH change of the solution. The small pH change can be calculated by determining the resulting change in the concentration of buffer components, i.e., a weak acid and its conjugate base or vice versa. The concentrations obtained using these stoichiometric calculations can be used to determine the solution’s final pH using the Henderson-Hasselbalch equation or an ICE table.
For example, a buffered solution contains 0.65 mol of formic acid and sodium formate. As the concentration of the weak acid and its conjugate base is the same here, the solution’s pH is equal to the pKa of the weak acid, which is 3.74 in this case. If 0.05 mol HNO3 is added into this solution, the resultant changes in the concentration of the formic acid and sodium formate can be determined by stoichiometric calculations as shown in the table below.
| H+ (aq) | HCOO− (aq) | HCOOH (aq) | |
| Before addition (M) | ~0.00 mol | 0.65 mol | 0.65 mol |
| Addition (M) | 0.050 mol | – | – |
| After addition (M) | ~0.00 mol | 0.60 mol | 0.70 mol |
The solution’s final pH can then be determined by plugging in changed concentrations of formic acid and sodium formate into the Henderson-Hasselbalch equation.
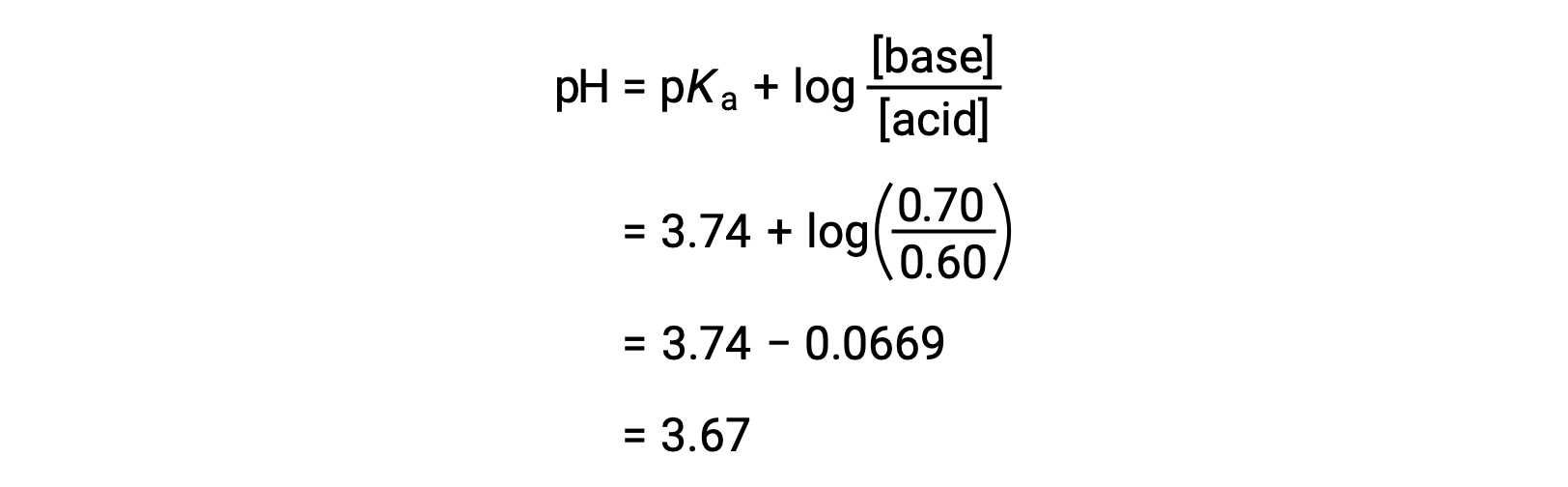
Thus, the addition of 0.05 mol of HNO3 reduces the pH of the solution from 3.74 to 3.67.
Similarly, if 0.10 mol NaOH is added into the same solution, the resultant changes in the concentration of the formic acid and sodium formate can be determined by stoichiometric calculations as shown in the table below.
| OH− (aq) | HCOOH (aq) | HCOO− (aq) | H2O (l) | |
| Before addition (M) | ~0.00 mol | 0.65 mol | 0.65 mol | - |
| Addition (M) | 0.10 mol | – | – | - |
| After addition (M) | ~0.00 mol | 0.55 mol | 0.75 mol | - |
The final pH of the solution can then be determined by plugging in changed concentrations of formic acid and sodium formate into the Henderson-Hasselbalch equation.
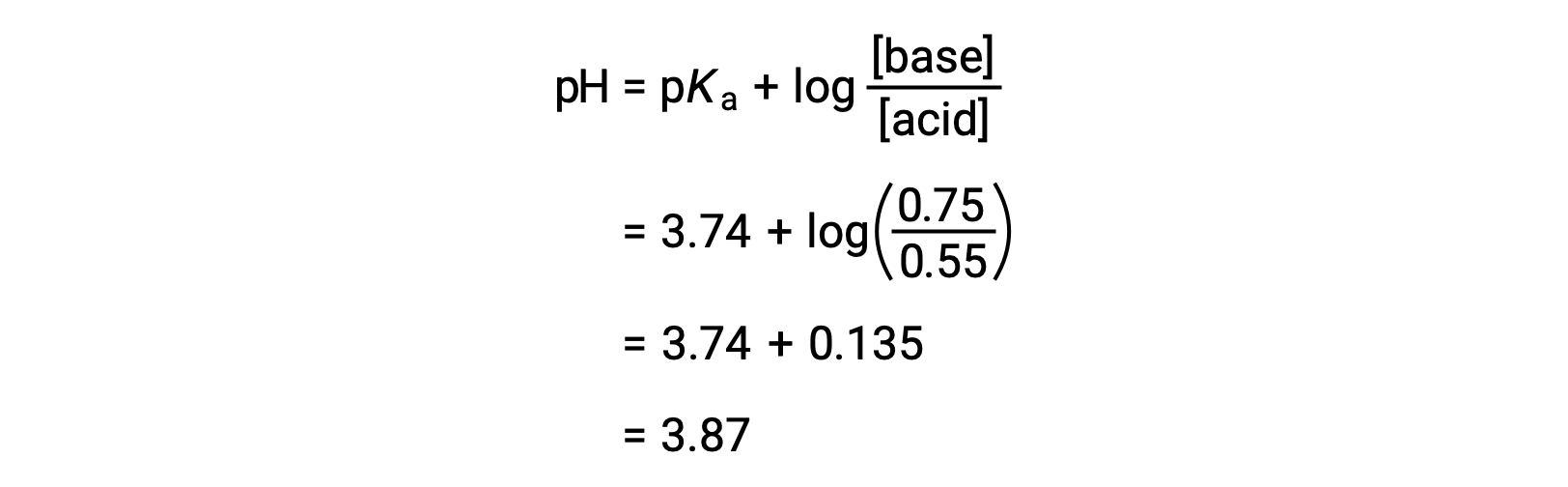
Thus, the addition of 0.10 mol NaOH increases the pH of the solution from 3.74 to 3.87.
