Dissection of Hippocampal Dentate Gyrus from Adult Mouse
Summary
A dissection technique for removal of the dentate gyrus from adult mouse under a stereomicroscope was demonstrated in this video-recorded protocol.
Abstract
Protocol
Dissection of hippocampal dentate gyrus
- In a deeply anesthetized mouse, carefully dissect the brain out from the skull and place it into ice-cold phosphate-buffered saline (PBS).
- In a Petri dish containing ice-cold PBS, cut the brain along the longitudinal fissure of the cerebrum using a surgical knife, and cut off the regions posterior to lambda (midbrain, hindbrain, and cerebellum).
- Place the cerebral hemisphere medial side up and, using forceps, carefully remove the diencephalon (thalamus and hypothalamus) under a dissection microscope. This will expose the medial side of the hippocampus, allowing for visualization of the dentate gyrus. The dentate gyrus is distinguishable from Ammon’s horn by the gaps between them. Injury to the hippocampus or surrounding area will make it more difficult to isolate the dentate gyrus.
- Insert a sharp needle-tip (e.g., 27-gauge needle) into each side of the dentate gyrus (boundaries of the dentate gyrus and Ammon’s horn; Figure 1), and slide the needles superficially along the septo-temporal axis of hippocampus to isolate the dentate gyrus.
- Pick up the isolated dentate gyrus using a needle or forceps and place it in a sample tube. The thus-obtained dentate gyrus tissue sample can be used immediately for any assay or stored in a deep-freezer for later use.
- Isolate the dentate gyrus from the other cerebral hemisphere using the same method.
Quantitative real-time PCR
The dentate gyrus was isolated using the above-mentioned method and the remaining hippocampus was dissected out as the Ammon’s horn sample from wild-type mice. Real-time PCR of beta-actin, TDO2, Dsp, Mrg1b and Tyro3 were performed with the dentate gyrus and the Ammon’s horn samples as described previously1. Primers 5′-CTGGCGAGATCACGATGACG and 5′-AAGCTACGCTGTTGTCTAACC were used for Mrg1b, and GCCTCCAAATTGCCCGTCA and 5′-CCAGCACTGGTACATGAGATCA for Tyro3.
Microarray analysis
Microarray experiments were performed with male wild-type mice and mice heterozygous for the alpha-isoform of calcium/calmodulin-dependent protein kinase II (alpha-CaMKII+/- mice) as described previously1. Briefly, RNA isolated from the whole hippocampus or dentate gyrus of wild-type and mutant mice was hybridized with a Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, CA), and each GeneChip was scanned by an Affymetrix GeneChip Scanner 3000 (GCS3000). GeneChip analysis was performed with Microarray Analysis Suite version 5.0.
Discussion
The dentate gyrus occupies approximately 25% to 30% of the volume of the hippocampal formation2,3. It has a unique cell composition and plays crucial roles in various brain functions. Therefore, techniques to isolate the dentate gyrus are useful for analyzing the events that occur specifically in this region.
Here, we demonstrated a procedure to efficiently dissect the dentate gyrus from adult mouse hippocampus and confirmed the precision of the technique. First, histologic study revealed that the dentate gyrus was separated without contamination by other regions (Figure 1), indicating that a pure dentate gyrus sample can be prepared.
Second, we confirmed that the dissected tissue is dentate gyrus by conducting real-time PCR of dentate gyrus-specific genes, TDO2 and Dsp, and Ammon’s horn enriched genes, Mrg1b and Tyro34 (Figure 2). The mRNA expressions of TDO2 (p=0.000023; n’s=4 and 4, respectively) and Dsp (p=0.0000030; n’s=4 and 4, respectively) in the dentate gyrus samples were detected at obviously higher levels, whereas Mrg1b (p=0.000080; n’s=4 and 4, respectively) and Tyro3 (p=0.00017; n’s=4 and 4, respectively) were lower levels, than those in the Ammon’s horn samples. Beta-actin expression levels did not differ in these samples (p=0.11; n’s=4 and 4, respectively). Thus, we could check whether or not the dentate gyrus was accurately dissected out by conducting such simple real-time PCR experiments.
Third, to assess the usefulness of this dissection method, we compared the mRNA expression level of whole hippocampus with that of dentate gyrus. Whole hippocampus and dentate gyrus obtained from wild-type (n’s=9 and 4, respectively) and alpha-CaMKII+/- mice (n’s=18 and 4, respectively) were processed for microarray analysis, and for all genes scored, the fold-change was calculated by dividing the mutant value by the wild-type value. The results indicated that the changes in mRNA expression, especially of dentate gyrus-specific molecules such as Dsp and TDO2, were detected with up to a 5-fold increase in sensitivity in dentate gyrus samples compared to whole hippocampal samples (Table 1). We previously demonstrated that alpha-CaMKII+/- mice exhibit behaviors related to human psychiatric disorders such as working memory deficits and an exaggerated infradian rhythm1,5. Furthermore, morphologic and electrophysiologic features of the dentate gyrus neurons in mutant mice are strikingly similar to those of immature dentate gyrus neurons in normal rodents, indicating that the neurons in these mutant mice fail to develop to maturity1. The immature dentate gyrus and down-regulated expression of Dsp and TDO2 mRNA in alpha-CaMKII+/- mice are consistent with the finding that Dsp and TDO2 can be used as markers of mature granule cells in the dentate gyrus (Ohira et al., unpublished data).
Taken together, this convenient and accurate dissection technique can be reliably used for studies focused on the dentate gyrus. Dentate gyrus tissue obtained using this method is applicable to other types of analyses as well, including proteomic and cell biology analyses.
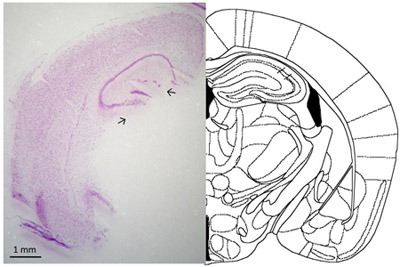
Figure 1. Verification of the isolated dentate gyrus by histologic study. A coronal section of the brain after isolating dentate gyrus was processed for Nissl staining (left panel), and a schematic diagram adapted from the mouse brain atlas6 represents the approximately the same level of the section shown in the left panel (right panel). Arrows indicate the directions of the needle-tip insertion. Scale bar, 1 mm.
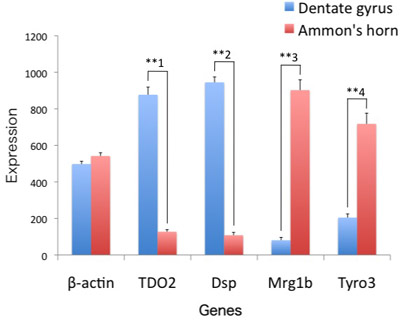
Figure 2. Verification of the isolated dentate gyrus by real-time PCR. The dentate gyrus and the Ammon s horn obtained from four wild-type mice were processed for real-time PCR of beta-actin, TDO2, Dsp, Mrg1b and Tyro3. Results are presented as means ± SEM. For statistical analysis, Student s t test was employed, and p values are followed: beta-actin, p=0.11; TDO2, p=0.000023 (**1); Dsp, p=0.0000030 (**2); Mrg1b, p=0.000080 (**3); and Tyro3, p=0.00017 (**4).
Table 1. Microarray analysis of whole hippocampus and dentate gyrus. Genes differentially expressed in dentate gyrus and whole hippocampus of alpha-CaMKII+/- mice were determined by calculating the fold-change from that detected in wild-type mice. Data were analyzed for statistical significance using the Student s t test between wild-type and alpha-CaMKII+/- mice. Among the genes whose expression exhibited p<0.05 in the dentate gyrus of alpha-CaMKII+/- mice compared to that of wild-type mice, the top 50 genes are listed. Note that the numbers of samples for dentate gyrus are much less than those for whole hippocampus. AffyID, Affymetrix probe identifier; CKII, alpha-CaMKII+/- mice; WT, wild-type mice.
| Dentate gyrus (p <0.05) WT: n=4, CKII+/-: n=4 |
Whole hippocampus WT: n=9, CKII+/-: n=18 |
|||||||
| Gene Title | Genebank | AffyID | Fold change | p value | Fold change | p value | ||
| desmoplakin | AV297961 | 1435494_s_at | 0.011018913 | 7.02694E-06 | 0.037021003 | 1.86126E-13 | ||
| desmoplakin | AV297961 | 1435493_at | 0.014369734 | 7.86747E-06 | 0.04232106 | 1.00579E-12 | ||
| tryptophan 2,3-dioxygenase | AI098840 | 1419093_at | 0.020986484 | 5.23546E-09 | 0.101037776 | 4.14823E-13 | ||
| nephronectin | AA223007 | 1452106_at | 0.075479901 | 1.05191E-08 | 0.234001154 | 1.66301E-15 | ||
| nephronectin | AA223007 | 1452107_s_at | 0.079457767 | 1.40433E-07 | 0.177974715 | 3.9758E-12 | ||
| thyrotropin releasing hormone receptor | M59811 | 1449571_at | 0.103105815 | 0.003093796 | 0.801412732 | 0.283994361 | ||
| ryanodine receptor 1, skeletal muscle | X83932 | 1427306_at | 0.104825517 | 3.38513E-07 | 0.650685017 | 0.000308462 | ||
| nescient helix loop helix 1 | NM_010916 | 1419533_at | 9.431896 | 6.7979E-06 | 4.078815314 | 5.27E-11 | ||
| copine family member IX | BB274531 | 1454653_at | 9.159157 | 7.99492E-06 | 1.797304153 | 0.000296375 | ||
| doublecortin-like kinase 3 | BB326709 | 1436532_at | 0.109336662 | 1.95278E-07 | 0.56697229 | 2.62633E-08 | ||
| calpain 3 | AF127766 | 1426043_a_at | 0.111269769 | 8.07053E-06 | 0.370956608 | 2.04421E-14 | ||
| Adult male corpus striatum cDNA, RIKEN full-length enriched library, clone:C030023B07 product:unclassifiable, full insert sequence | BB357628 | 1460043_at | 0.118712341 | 6.16926E-07 | 0.682339204 | 2.33001E-06 | ||
| collagen and calcium binding EGF domains 1 | AV264768 | 1437385_at | 0.124043978 | 3.65669E-05 | 0.488394112 | 4.05538E-06 | ||
| amyloid beta (A4) precursor protein-binding, family A, member 2 binding protein | AK013520 | 1431946_a_at | 7.7986307 | 1.2098E-06 | 2.099164713 | 1.67047E-06 | ||
| calbindin-28K | BB177770 | 1456934_at | 0.130255444 | 3.32186E-06 | 0.572605751 | 1.99157E-10 | ||
| Transcribed locus | AV328597 | 1443322_at | 0.133290835 | 5.43583E-06 | 0.562767164 | 7.56544E-06 | ||
| neuropeptide Y receptor Y2 | NM_008731 | 1417489_at | 0.135319609 | 0.000113407 | 0.781498474 | 0.00394504 | ||
| ras responsive element binding protein 1 | BE197381 | 1428657_at | 0.138235114 | 7.93691E-07 | 0.651220705 | 2.94209E-05 | ||
| glial cell line derived neurotrophic factor family receptor alpha 2 | BB284482 | 1433716_x_at | 0.139062563 | 2.35371E-06 | 0.669544709 | 0.000214146 | ||
| preproenkephalin 1 | M13227 | 1427038_at | 6.9850435 | 2.39074E-08 | 1.766018828 | 0.000250501 | ||
| RIKEN cDNA 1810010H24 gene | BI729991 | 1428809_at | 6.8658915 | 1.88516E-05 | 2.77573142 | 6.81865E-09 | ||
| ryanodine receptor 1, skeletal muscle | BG793713 | 1457347_at | 0.151364292 | 3.35612E-05 | 0.503144617 | 4.32907E-05 | ||
| protocadherin 21 | NM_130878 | 1418304_at | 0.152671849 | 8.57783E-06 | 0.670714726 | 1.56309E-05 | ||
| cornichon homolog 3 (Drosophila) | NM_028408 | 1419517_at | 0.153724144 | 8.90755E-06 | 0.95780695 | 0.661055608 | ||
| harakiri, BCL2 interacting protein (contains only BH3 domain) | BQ175572 | 1439854_at | 0.154284407 | 2.0118E-05 | 0.56516812 | 4.86925E-09 | ||
| carbohydrate (N-acetylgalactosamine 4-0) sulfotransferase 9 | AK017407 | 1431897_at | 0.155238951 | 5.37423E-06 | 1.14910007 | 0.215637733 | ||
| calpain 3 | AI323605 | 1433681_x_at | 0.160871988 | 1.07655E-05 | 0.477164757 | 1.33753E-11 | ||
| zinc finger, CCHC domain containing 5 | BQ126004 | 1437355_at | 0.161812078 | 3.08262E-06 | 0.421252632 | 0.01152969 | ||
| loricrin | NM_008508 | 1448745_s_at | 0.165129967 | 1.86362E-05 | 0.639733409 | 0.000729772 | ||
| spondin 1, (f-spondin) extracellular matrix protein | BC020531 | 1451342_at | 0.168035879 | 6.67867E-07 | 0.821042412 | 0.023650765 | ||
| RIKEN cDNA A930035E12 gene | AV348640 | 1429906_at | 5.9086795 | 1.747E-07 | 1.470383201 | 0.104085454 | ||
| BB247294 RIKEN full-length enriched, 7 days neonate cerebellum Mus musculus cDNA clone A730018G18 3′, mRNA sequence. | BB247294 | 1447907_x_at | 5.9047494 | 1.04931E-05 | 1.968147585 | 0.00010636 | ||
| FERM domain containing 3 | BB099015 | 1437075_at | 5.860216 | 0.000345581 | 2.780297178 | 1.83072E-06 | ||
| neuronal pentraxin 2 /// hypothetical protein LOC100044234 | NM_016789 | 1420720_at | 5.7568517 | 1.34227E-06 | 2.652516957 | 0.000206279 | ||
| Transcribed sequences | BG076361 | 1460101_at | 5.657735 | 2.5015E-06 | 1.296248831 | 0.22870031 | ||
| spondin 1, (f-spondin) extracellular matrix protein | BC020531 | 1424415_s_at | 0.17783576 | 1.01658E-06 | 0.836181248 | 0.001380141 | ||
| calbindin-28K | BB246032 | 1448738_at | 0.180317904 | 1.35961E-05 | 0.647334052 | 4.12268E-09 | ||
| MARCKS-like 1 | AV110584 | 1437226_x_at | 0.186235935 | 1.47067E-06 | 0.499291387 | 2.34984E-08 | ||
| matrilin 2 | BB338441 | 1455978_a_at | 0.187783528 | 6.19122E-05 | 0.8967688 | 0.282337853 | ||
| matrilin 2 | BC005429 | 1419442_at | 0.188195795 | 0.000105295 | 0.915528892 | 0.35282097 | ||
| spondin 1, (f-spondin) extracellular matrix protein | BQ175871 | 1442613_at | 0.189956563 | 9.41195E-06 | 0.861033222 | 0.1394266 | ||
| arrestin 3, retinal | NM_133205 | 1450329_a_at | 5.2130346 | 2.90599E-05 | 3.944218329 | 1.07437E-07 | ||
| RIKEN cDNA A330050F15 gene | AV325555 | 1457558_at | 0.19186781 | 0.000119342 | 0.660282035 | 2.47342E-05 | ||
| contactin 3 | BB559510 | 1438628_x_at | 0.194404608 | 4.08641E-07 | 0.918742591 | 0.022545297 | ||
| calbindin-28K | BB246032 | 1417504_at | 0.196381321 | 2.24182E-05 | 0.619305124 | 3.6222E-06 | ||
| gastrin releasing peptide | BC024515 | 1424525_at | 4.9436426 | 3.00588E-05 | 2.752845903 | 5.72954E-07 | ||
| sortilin-related VPS10 domain containing receptor 3 | AK018111 | 1425111_at | 4.885766 | 1.03645E-05 | 1.29051599 | 0.029733649 | ||
| dopamine receptor D1A | BE957273 | 1455629_at | 4.869493 | 3.77525E-05 | 1.815881979 | 0.000516498 | ||
| proprotein convertase subtilisin/kexin type 5 | BB241731 | 1437339_s_at | 0.210528027 | 7.83039E-05 | 0.574126078 | 9.15496E-05 | ||
| interleukin 1 receptor, type I | NM_008362 | 1448950_at | 0.210572243 | 9.64524E-06 | 0.241135352 | 2.79816E-08 | ||
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Yoko Nabeshima at Kyoto University for her instruction on the dissection technique and Ms. Aki Miyakawa at Fujita Health University for her support to film. This work was supported by the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, a Grant-in-Aid for Scientific Research on Priority Areas -Integrative Brain Research (Shien)- from MEXT in Japan, and by a Grant-in-Aid from CREST of the Japan Science and Technology Agency.
References
- Yamasaki, N., Maekawa, M., Kobayashi, K., Kajii, Y., Maeda, J., Soma, M., Takao, K., Tanda, K., Ohira, K., Toyama, K., Kanzaki, K., Fukunaga, K., Sudo, Y., Ichinose, H., Ikeda, M., Iwata, N., Ozaki, N., Suzuki, H., Higuchi, M., Suhara, T., Yuasa, S., Miyakawa, T. Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mol. Brain. 1, 6-6 (2008).
- Insausti, A. M., Megias, M., Crespo, D., Cruz-Orive, L. M., Dierssen, M., Vallina, I. F., Insausti, R., Florez, J. Hippocampal volume and neuronal number in Ts65Dn mice: a murine model of Down syndrome. Neurosci. Lett. 253, 175-175 (1998).
- Redwine, J. M., Kosofsky, B., Jacobs, R. E., Games, D., Reilly, J. F., Morrison, J. H., Young, W. G., Bloom, F. E. Dentate gyrus volume is reduced before onset of plaque formation in PDAPP mice: a magnetic resonance microscopy and stereologic analysis. Proc. Natl. Acad. Sci. U.S.A. 100, 1381-1381 (2003).
- Lein, E. S., Zhao, X., Gage, F. H. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J. Neurosci. 24, 3879-3879 (2004).
- Matsuo, N., Yamasaki, N., Ohira, K., Takao, K., Toyama, K., Eguchi, M., Yamaguchi, S., Miyakawa, T. Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front. Behav. Neurosci. 3, 20-20 (2009).
- Franklin, K. B. J., Paxinos, G. . The Mouse Brain in Stereotaxic Coordinates. , (1997).

