Dopamine Release at Individual Presynaptic Terminals Visualized with FFNs
Summary
A new means to measure neurotransmission optically using fluorescent dopamine analogs.
Abstract
The nervous system transmits signals between neurons via neurotransmitter release during synaptic vesicle fusion. To observe neurotransmitter uptake and release from individual presynaptic terminals directly, we designed fluorescent false neurotransmitters as substrates for the synaptic vesicle monoamine transporter. Using these probes to image dopamine release in the striatum, we made several observations pertinent to synaptic plasticity. We found that the fraction of synaptic vesicles releasing neurotransmitter per stimulus was dependent on the stimulus frequency. A kinetically distinct “reserve” synaptic vesicle population was not observed under these experimental conditions. A frequency-dependent heterogeneity of presynaptic terminals was revealed that was dependent in part on D2 dopamine receptors, indicating a mechanism for frequency-dependent coding of presynaptic selection.
Hui Zhang and Niko G. Gubernator contributed equally to this work.
Protocol
This method was used in the research reported in Gubernator et al., Science 324 (5933). 1441-1444 (2009).
1. Preparing acute striatal slices
- Before preparing acute striatal slices, one must oxygenate the artificial cerebrospinal fluid (ASCF) and chill it with ice for at least 15 minutes prior to the brain extraction. Keep the ACSF ice cold and oxygenated during the dissection. ACSF (in mM): NaCl 125, KCl 2.5, NaHCO3 26, CaCl2 2.4, MgSO4 1.3, KH2PO4 0.3, glucose 10, HEPES 5; pH 7.3-7.4, 290-295 mOsm.
- Decapitate male mouse without anesthesia.
- Extract the whole brain from the mouse, and mount it on the vibratome tray placed on the vibratome using Krazy®Glue. Brain extraction and mounting must be done within 2 minutes. During this time, make sure to keep the ACSF ice cold and oxygenated to keep the tissue healthy.
- Cut coronal striatal brain slices at 250μm thickness between bregma +1.54 to +0.621. Three whole slices will be obtained which can then be cut into 6 half slices with a needle.
- Incubate the brain slices in oxygenated ACSF at room temperature for at least 1 hour before probe loading. Slices display good FFN511 loading and remain active for imaging up to 4 hours after slice preparation.
2. Loading FFN511
- Prepare FFN511 loading solution (10μM FFN511 in ACSF); also prepare 100μM ADVASEP-7 in ACSF. All solutions should be prepared freshly and oxygenated at least 15 minutes prior to loading into the slices.
- Individually incubate slice with FFN511 loading solution for 30 minutes at room temperature.
- Then remove the dye bound to extracellular tissue by incubating the loaded slice in oxygenated 100μM ADVASEP-7 ACSF for 30 minutes at room temperature2. Slices are now ready for imaging.
3. Imaging FFN511 in the brain slices
Imaging of FFN511 in the slices is performed using multiphoton laser scanning microscope. We are using a Prairie Ultima multiphoton laser scanning microscope (We were using a Zeiss LSM 510 NLO multiphoton laser scanning microscope previously and some results were obtained with the Zeiss microscope).
- Place the FFN511-loaded slice in the recording chamber (RC-27L, Warner) and superfuse with oxygenated ACSF at a flow rate of 1-2 ml per minute. Then place a platinum harp with nylon strings on top of the slice to minimize movement during the experiment. To further minimize movement, allow the slice to stabilize for at least 10 minutes in the chamber prior to imaging.
- Visualize and locate the dorsal striatum with bright field luminescence under a 10x water immersion objective.
- Place and position the bipolar twisted tungsten electrodes in this region if performing a stimulation experiment. The ideal imaging area is located within 300 μm in between the two tips of the stimulating bipolar electrode. Place this region in the center of the visual field.
- Image FFN511-labeled striatal terminals under a 63x (0.9 NA) water immersion ultraviolet objective. FFN511 is excited at 760 nm with a Mai Tai laser from Spectral Physics and optimal fluorescence of striatal terminals is seen through a band pass filter (480-520 nm). Images are captured in 12-bit format with 75 x 75 μm regions of interest at 512 x 512 pixel resolution. For stimulated destaining experiments, to compensate for z-axis shift, a z-series of 5-7 images, separated by 1 μm in the z-plane, is obtained for each time period.
4. FFN511 Destaining
The FFN511 label can be destained either by high concentration of KCl (we use 70 mM KCl), (+)-amphetamine sulphate (AMPH, 20 μM), or electrical stimulation. In the KCl destaining experiment, when a high potassium ACSF solution is applied to the brain slice, the FFN511 label destains within 2 minutes. Record xyz-t images to track FFN511 destaining over time. The following describes electrical stimulation-dependent dopamine terminal destaining:
- To track FFN511 destaining over time, xyz-t images are recorded. To ensure minimal movement of the slice (less than 4 μm in z-axis) and no photobleaching by the laser, control time series images of the z-stack should be obtained for at least 5 minutes prior to stimulation. Otherwise, adjust the laser power.
- Following these control images, continue the xyz-t imaging while start the stimulation at frequencies of 1, 4 or 20 Hz. Stimuli at 1, 4 or 20 Hz (300 μs x 1 mA) are applied to the striatum locally by an Iso-Flex stimulus isolator triggered by a Master-8 pulse generator (AMPI, Jerusalem, Israel) using bipolar electrodes. To minimize the variation in the depolarization of release sites, we have used a stimulation protocol applying 150% of the maximal stimulation intensity determined by cyclic voltammetry.
- Use proper software for imaging analysis. In our lab, we use Image J (Wayne Rosband, National Institutes of Health, Rockville, MD) and custom-written software in IDL (Research Systems, Boulder, CO) to quantify the puncta fluorescence and the changes with time3.
Represenative Results
Figure 1 shows FFN511 loading into striatal slice is now complete. Representative images using 10x and 60x objective are shown in Fig.1. The selective labeling of dopamine terminals can be destained by 20 μM amphetamine.
Figure 2 shows destaining of FFN511 labeling by high KCl.
Figure 3 shows
destaining of FFN511 labeling by local electrical stimulation.
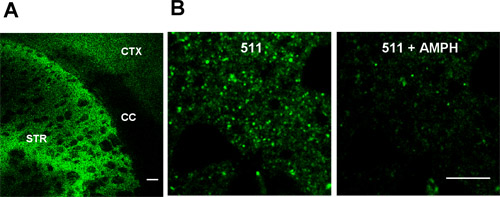
Figure 1. FFN511 labels dopamine terminals in live cortical-striatal acute slices. (A) Labeling by FFN511 in acute live cortical-striatal slice: abundant labeling in the striatum (STR), sparser labeling in cortex (CTX), and no label in corpus callosum (CC). Scale bar: 100 μm. (B) Destaining of FFN511 from the striatum by amphetamine. Left panels: before amphetamine; right panels: after 20 minutes of 20 μM amphetamine. Scale bar: 10 μm.
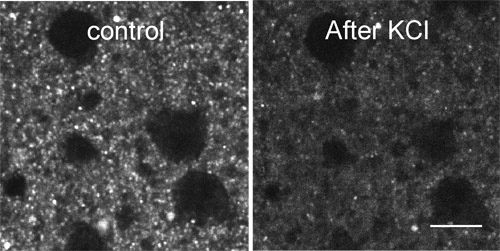
Figure 2. Destaining of FFN511 labeling by high KCl. FFN511 labeling is destained within 2 min application of 70 mM KCl in ACSF. Scale bar: 10μm.
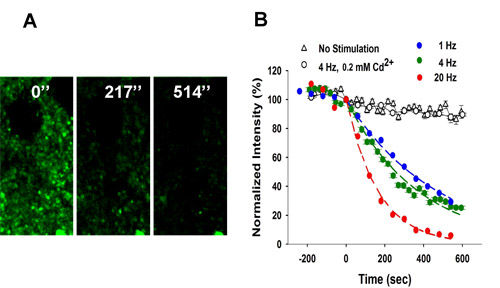
Figure 3.
Frequency-dependent destaining of FFN511 labeling in striatum. (A) Local stimulation at 4 Hz resulted in destaining from the terminals. Stimulation began at t = 0. Scale bar: 5 μm. (B) Destaining of FFN511 at 4 Hz is Ca2+– and frequency-dependent. Controls received no stimulation (153 puncta from 3 slices). Destaining with cadmium chloride (200 μM) was identical to unstimulated controls (475 puncta from 5 slices). The destaining curves for each stimulation frequency were fit by a single exponential decay function and half-life (t1/2) values calculated as τ x 0.693 (1 Hz: 765 puncta from 9 slices, 4 Hz: 410 puncta from 7 slices, 20 Hz: 416 puncta from 6 slices). Please refer to the video micrograph from 2-photon microscopy of FFN511 destaining during exocytosis in a striatal brain slice preparation.
Discussion
In this video, we demonstrate a method to visualize neurotransmission optically using fluorescent dopamine analogs. FFN511 is the first generation of FFNs we have developed. Although it was designed by targeting the neuronal vesicular monoamine transporter (VMAT2) that carries monoamine neurotransmitters from the cytoplasm into synaptic vesicles, and specifically labels dopamine terminals in the striatum and presumed catecholamine and/or serotonin terminals in the cortex (as shown in the Science paper), an appropriate loading period is critical for the specificity since FFN511 is relatively hydrophobic. We found that incubation for longer than 40 minutes will result in extensive nonspecific staining in striatal slices. The specificity can be straightforwardly determined by destaining using a high concentration of KCl, which should cause the release of FFN within functional synaptic vesicles. Exposure of the slice to FFN511 for less than 15 minutes will result in weak fluorescent signal due to insufficient loading of the dye in the terminals. In this protocol, we use 100μM ADVASEP-7 to remove the dye bound to extracellular tissue. This step is not necessary, but if it is omitted, the washout time in ACSF needs to be prolonged. In summary, depending on the preparation you chose, you will need to modify the concentration and loading period to determine optimal labeling.
Disclosures
The authors have nothing to disclose.
Acknowledgements
D. Sames thanks The G. Harold & Leila Y. Mathers Charitable Foundation and Columbia University’s Initiatives in Science and Engineering.
D. Sames and D. Sulzer thank the McKnight Foundation for The McKnight Technological Innovations in Neuroscience Award
D. Sulzer thanks NIDA, NIMH, and the Picower and Parkinson’s Disease Foundations.
H. Zhang thanks NARSAD.
R.H. Edwards thanks the Michael J. Fox Foundation, the National Parkinson
Foundation, NIDA and NIMH.
We thank Robert Burke for 6-OHDA injections and advice, Mark Sonders for useful discussion, Merek Siu for imaging analysis programming, and Jan Schmoranzer for technical support with the TIRF microscopy setup.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| FFN511 (8-(2-Amino-ethyl)-2,3,5,6-tetrahydro-1H,4H-11-oxa-3a-aza-benzo[de]anthracen-10-one) | Dalibor Sames’s laboratory at Columbia University | |||
| ADVASEP-7 | CyDex, Overland Park, KS | AR-OA7-005 | ||
| RC-27L Recording chamber | Warner Instrument | 64-0375 | ||
| PELCO PrepEze 6-Well Holder | Ted Pella, Inc. | 36157-1 | For slice incubation | |
| Master-8 pulse stimulator and Iso- Flex stimulus isolator | AMPI, Jerusalem, Israel |
References
- Franklin, K. B. J., Paxinos, G. . The mouse brain in stereotaxi coordinators. , (1997).
- Kay, A. R. Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey, and rat. Neuron. 24, 8098-8117 (1999).
- Bamford, N. S. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 42, 653-653 (2004).

