Platelet Adhesion and Aggregation Under Flow using Microfluidic Flow Cells
Summary
The platelet adhesion cascade takes place in the presence of shear flow, a factor not accounted for in conventional (static) well-plate assays. This article reports on a platelet-aggregation assay utilizing a microfluidic well-plate format to emulate physiological shear flow conditions.
Abstract
Protocol
Part 1: Preparation of the microfluidic channels of a BioFlux plate.
- Before running the flow cell experiment, one needs to prepare the microfluidic channels with a protein coating of interest. In this case, collagen I will be used. Each channel has an inlet and an outlet well. For this plate, the inlet well is the left well feeding the channel and the outlet well is on the right.
- Dilute the collagen I (5mg/ml stock) coating to 200μg/ml concentration in 0.02M acetic acid. One will need 20μl for each channel used. Mix by gentle triteration with a micropipette tip.
- Add 20 μl of coating to each respective channel to be used. One must dispense the liquid into to inner punch of the outlet well feeding the microfluidic channel of interest using a micropipette. Avoid introducing bubbles, do not push the air out of the pipette. Include one channel without collagen for a no collagen control (a negative control for adhesion and aggregation).
- Attach the interface to the plate by first finger tightening the 4 screws and then once they are all set, use the torque driver to completely tighten. The torque driver will click when it reaches the maximum tightness.
- Using manual mode in the BioFlux software, apply perfusion to the channels of interest at 2dyn/cm2—from the outlet well. Here one must watch for the opposite inner punch to be filled with liquid. This will take several minutes. One can either watch this occur using a low power objective on a microscope (4X) and finding the inlet well or by holding the plate and looking into the inlet wells from below. One should first see a tiny bead of liquid that slowly fills the inner punch. Once the inlet inner punch is filled, stop perfusion at once by pushing stop in the software.
- Incubate the plate at room temperature for one hour.
- Remove the interface and add 1 ml of PBS (plus Ca2+/Mg2+) to the outlet well. Start perfusion from the outlet well at 2dyn/cm2. Continue perfusion for 10 minutes. Stop perfusion and remove the interface.
- Remove excess PBS from both inlet and outlet wells – never remove liquid that fills the inner punch. Block the channels using blocking solution ( 0.5% v/v BSA in PBS (plus Ca2+/Mg2+)). Add 1 ml of blocking solution to each outlet well to be used and perfuse from the outlet well at 2dyn/cm2. Continue perfusion for 10 minutes. Stop perfusion and remove the interface. Channels prepared up to this step can be used throughout the day.
- Prior to adding blood, remove the liquid on both sides of the channel except for the inner punches.
Part 2. Preparing the blood with GPIIb/IIIa inhibitory antibody.
- While the collagen is incubating in the channels, one must prepare the whole blood. One should follow the biosafety regulations dictated by his/her institute when handling human blood.
- Fresh human blood (from a fasting individual) drawn into sodium citrate anticoagulant should be used within 3 hours of collection.
- Prepare blood by adding 4μM calcein AM. Prepare a 4mM stock of calcein AM in DMSO and add to blood at a dilution of 1/1000 v/v. Mix by gentle inversion.
- Dispense 1 ml of the calcein AM labeled blood into 10 – 1.5mL microtubes. Add GPIIb/IIIa inhibitor antibody to each at the desired dilution (the molecular weight of IgG is ~150kDa). An example dilution series is from 1200nM to 9nM and includes a no antibody control and an unrelated antibody control. An excellent positive control for inhibition would be ReoPro (abciximab, Eli Lilly) at a concentration of 20ug/ml. Mix by gentle inversion.
- Incubate at room temperature for 1 hour. Mix by gentle inversion every 10 minutes.
Part 3: Running the flow cell experiment on the BF1000 workstation.
- First, one must set up an automated protocol in the BioFlux control module. For collagen I, one should set up a protocol to run 10dyn/cm2 for 10 minutes from the outlet well. At this point, one should also set up data acquisition parameters using the BF1000 workstation, including channel positions, capture in FITC wavelength and time-lapse information. It is recommended to capture 3 fields of view using a 10x objective per channel every 30 seconds over a duration of 10 minutes total.
- Working quickly, place 500ul of prepared blood of each condition into separate prepared channels. Place no antibody control blood into a channel that is coated with collagen and one that is just blocked.
- Place the interface on the plate.
- Place the plate on the BF1000 workstation microscope. Clamp on the interface.
- Perform plate load adjustment. Also, move stage to one well containing blood. Set FTIC image capture parameters (exposure time, gain etc…).
- In the BioFlux montage software, start acquisition to begin flow and acquisition simultaneously.
- Remove the plate from BF1000 workstation, dispose of plate according to institutional guidelines.
Part 4: Representative Results.
Here the protocol for platelet adhesion and aggregation in microfluidic channels was presented. Treatment with a platelet aggregation inhibitor, anti-GPIIb/IIIa was also included in the protocol. Using collagen coated microfluidic channels of the BioFlux system, one should expect to see aggressive thrombus formation over time with the untreated control blood sample and no thrombus formation on the uncoated channel. In one recently performed experiment, the average size of aggregates under control conditions was 2000μm2.
Figure 1.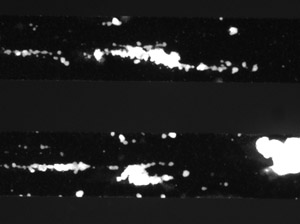
Activated GPIIb/IIIa is potent mediator of platelet-platelet interactions and aggregation stabilization1. Adhesion to collagen activates the GPIIb/IIIa complex to elicit this response 2. After incubation with anti-GPIIb/IIIa for 1 hour prior to shear exposure, one should expect to observe a decrease in size of thrombi as well as a decrease in the frequency of thrombus formation. A dose dependent response can typically be observed at 10dyn/cm2.
Figure 2.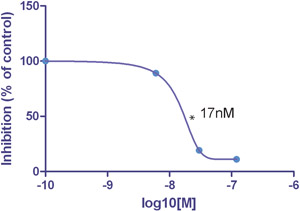
The IC50 value for this particular inhibitor was 17nM at 10 dyn/cm2. Maximum inhibition (for this donor) compared to the no antibody control was 11% at 10 dyn/cm2.
While the methods here were presented using a specific extracellular matrix protein and a specific inhibitor of platelet aggregation, the protocol is extensible to other coatings, other cell types and other inhibitors for cell adhesion assays. The key ingredients to success of such experiments are avoidance of bubbles in the microfluidic channels, correct dilution of all reagents in the correct diluents, and appropriate incubation conditions for all reagents.
Discussion
While the methods here were presented using a specific extracellular matrix protein and a specific inhibitor of platelet aggregation, the protocol is extensible to other coatings, other cell types and other inhibitors for cell adhesion assays. The key ingredients to success of such experiments are avoidance of bubbles in the microfluidic channels, correct dilution of all reagents in the correct diluents, and appropriate incubation conditions for all reagents.
Disclosures
The authors have nothing to disclose.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| collagen I, Bovine | Reagent | Invitrogen | A1064401 | other sources of collagen I can be used |
| PBS plus Ca/Mg | Reagent | Invitrogen | 14040-117 | |
| Bovine serum albumin (BSA) | Reagent | EMD | EM-2930 Bottom of Form | From VWR |
| calcein AM | Reagent | Invitrogen | C1430 | Calcein AM from BD can also be used |
| BioFlux 1000 System | Reagent | Fluxion Biosciences | Contact Company | |
| BioFlux Plate | Reagent | Fluxion Biosciences | 900013 | |
| antiGPIIb/IIIa | Reagent | Abcam | ab33407 | an alternative # is ab15021 |
| ReoPro | Reagent | Eli Lilly | by Rx only |
References
- Jackson, S. P. The growing complexity of platelet aggregation. Blood. 109, 5087-5095 (2007).
- Nakamura, T., Kambayashi, J., Okuma, M., Tandon, N. N. Activation of the GP IIb-IIIa complex induced by platelet adhesion to collagen is mediated by both alpha2beta1 integrin and GP. VI. J Biol Chem. 274, 11897-11903 (1999).

