Drosophila melanogaster Ovary Dissection: A Technique for Ex Vivo Tissue Preparation
Abstract
Source: Parker, D. J., et al. Studying Mitochondrial Structure and Function in Drosophila Ovaries. J. Vis. Exp. (2017).
This video describes the general anatomy of the Drosophila ovary and features a method of dissecting ovaries for live or fixed imaging.
Protocol
This protocol is an excerpt from Parker et al., Studying Mitochondrial Structure and Function in Drosophila Ovaries, J. Vis. Exp. (2017).
1. Preparation of Drosophila (the tools required are depicted in Figure 1A)
- For any of the experiments described, collect Drosophila (maintained at room temperature, or 25 °C) within 5 days of eclosion and place them in a vial filled with 5 – 7 mL of Drosophila food (see Table of Materials), with no more than 25 flies in each vial; maintain a female:male ratio of 2:1.
- Sprinkle a small amount of granulated yeast to stimulate Drosophila egg production. Perform experimental manipulation within 2 – 4 days.
2. Dissection of Drosophila Ovaries (the tools required are depicted in Figure 1A)
- Warm insect dissecting medium (see Table of Materials) to room temperature, 25 °C. Fill three wells of an eight-well glass dissecting dish, with 200 µL of medium in each well.
- Anesthetize Drosophila with CO2 by placing the needle of the blow gun under the vial plug. Place them on a fly pad. Using a dissecting microscope, sort out 5 females and place them in the first well of the dissecting dish. Handle one Drosophila at a time when performing live microscopy.
- While looking through the eyepiece of the dissection microscope, sever the thorax from the abdomen using two pairs of forceps. Using the forceps, carefully transfer the abdomens to the second well of the dish.
- Use one pair of forceps to hold the abdomen at the posterior end, and slowly push the ovaries out (along with the other abdominal contents) with the other pair of forceps. Should this attempt fail, carefully remove the abdominal exoskeleton by inserting the forceps into the anterior end to release the ovaries.
- Using the forceps, hold an individual ovary by the opaque posterior end (i.e., the yolk-filled, late-stage eggs) and move it carefully to the third well of the dish for teasing to process it for live microscopy or for fixing to perform immunostaining.
- Carefully tease the protective sheath from around the ovaries by sweeping a teasing needle lightly from the posterior to the anterior end of each ovary while holding it by the posterior end with a pair of forceps.
NOTE: To minimize damage during teasing, bend the needle tip and zoom in on each ovary by increasing the magnification of the microscope (Figure 1A). Teasing should be effective enough to break the sheath, but it should also be carefully done to preserve the integrity of the ovarioles.
Representative Results
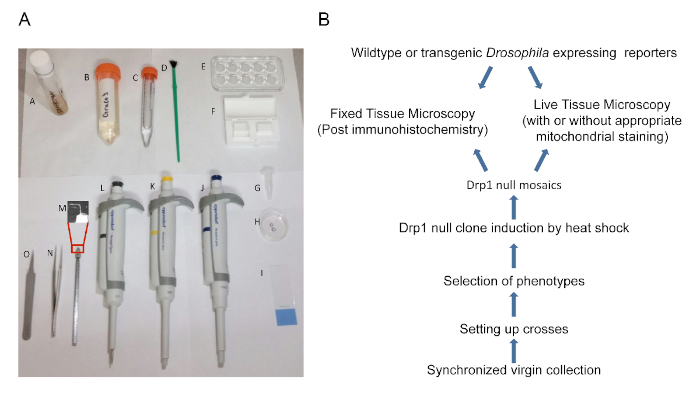
Figure 1: Experimental Plan and Preparation. (A) Tools used in the methods described: A. Fly vial; B. Insect dissecting medium; C. Paraformaldehyde; D. Fly brush; E. Dissecting dish; F. Cover glass; G. Microfuge tube. H. Glass-bottomed dish; I. Glass slide; J. 1,000-µL micropipette; K. 200-µL micropipette; L. 2.5-µL micropipette; M. Teasing needle with a bent tip (tip is magnified); N. Thick forceps; O. Thin forceps. (B) A flow chart schematic representing the methods described. Please click here to view a larger version of this figure.
Materials
| Grace's Media (Insect Dissecting Medium) | Fisher Scientific | 30611031-2 | |
| 41 Paraformaldehyde AQ | Electronic Microscopy Sciences | 50-259-99 | |
| PolyLysine | MP Biomedicals | ICN15017625 | |
| Fly Vials | Fisher Scientific | AS-515 | |
| Fly Vial Plugs | Fisher Scientific | AS273 | |
| Jazzmix Drosophila food (Drosophila food) | Fisher Scientific | AS153 | |
| Azer Scientific EverMark Select Microscope Slides | Fisher Scientific | 22-026-252 | |
| Microscope Cover Glass | Fisher Scientific | 12-542-B | |
| Mat Tek Corp Glass Bottom Mircrowell Dish | Fisher Scientific | P35G-0-14-C | |
| Active Dried Yeast | Fisher Scientific | ICN10140001 | |
| Dumont #5 Forceps | Fine Science Technologies | 11251-20 | |
| Moria Nickel Plated Pin Holder | Fine Science Technologies | 26016-12 | |
| Minutien Pins | Fine Science Technologies | 26002-15 | |
| MYFP ( w[*]; P{w[+mC]=sqh-EYFP-Mito}3 ) | Bloomington Stock Center | 7194 | |
| Fly Pad | Fly stuff | 59-118 | |
| Blowgun | Fly stuff | 54-104 | |
| Blowgun needle | Flystuff | 54-119 | |
| Dissecting Microscope | Carl Zeiss | Stemi 2000 |

