Lifespan Protocol for Drosophila melanogaster: Generating Survival Curves to Identify Differences in Fly Longevity
Abstract
Source: Bovier, T. F., et al. Methods to Test Endocrine Disruption in Drosophila melanogaster. J. Vis. Exp. (2019).
Scientists use the Drosophila lifespan protocol to measure how different experimental conditions affect flies' longevity and generate survival curves in the process to quantify the experimental outcomes. The sample protocol features the assay being applied to study the effects on longevity from exposure to varying concentrations of endocrine disruptor chemicals, or EDCs, such as 17-α-ethinylestradiol (EE2).
Protocol
This protocol is an excerpt from Bovier et al., Methods to Test Endocrine Disruption in Drosophila melanogaster, J. Vis. Exp. (2019).
1. Food Preparation
- For stock maintenance and for larval growth, use a cornmeal medium containing 3% powdered yeast, 10% sucrose, 9% precooked cornmeal, 0.4% agar, thereafter called Cornmeal medium (CM).
- Put 30 g of yeast into 100 mL of tap water, bring it to a boil and let it boil for 15 min.
- Separately, mix well 90 g of precooked cornmeal, 100 g of sugar, and 4 g of agar into 900 mL of tap water.
- Bring the solution to a boil, lower the heat and cook for 5 minutes stirring continuously.
- After 5 minutes, add the hot yeast solution and simmer for another 15 min.
- Turn off the heat source and allow the solution to cool to about 60 °C.
- Add 5 mL/L of 10% methyl 4-hydroxybenzoate in ethanol, mix thoroughly and let it sit for 10 min.
NOTE: Be careful with the amount of methyl 4-hydroxybenzoate, given that a high concentration of fungicide could be lethal for larvae. - Dispense the medium into vials/bottles: 8 mL into each fly vial (25 mm x 95 mm), 3 mL into each fly vial (22 mm x 63 mm) and 60 mL into each fly bottle (250 mL).
- Cover vials with cheesecloth and allow them to dry at room temperature (RT) for 24 h prior to storage.
- Calibrate experimentally right consistency and hydration of the CM by modifying either the amount of agar used and/or the cooling/drying times.
NOTE: unplugged, boxed and wrapped vials are stable for about 15 days at 4 °C.
- For Drosophila adults, use a medium containing 10% powdered yeast, 10% sucrose, 2% agar, thereafter called adult medium (AM).
- Mix 10 g of powdered yeast, 10 g of sucrose, 2 g of agar into 100 mL of distilled water.
- Bring this mixture to a boil two times, with a 3 minutes interval, or until agar is dissolved, by using a microwave.
- Once the solution cools to 60 °C, add 5 mL/L of 10% Methyl 4-hydroxybenzoate in ethanol, mix thoroughly and dispense in vials (10 mL per vial).
- Cover vials with cheesecloth and let them dry at RT for 24 h before storing.
NOTE: unplugged, boxed and wrapped vials are stable for about 15 days at 4 °C.
2. Drosophila EDC Dosing
- Prepare an appropriate stock solution dissolving the selected EDC in the suitable solvent. For the EE2 (molecular weight 296.403), dissolve 1.48 g in 10 mL of 100% ethanol to make a 0.5 M stock solution and store at -80 °C.
CAUTION: EDCs are considered environmental pollutant and precautions should be taken in handling them. - Dilute the EE2 stock solution in 10% ethanol in water (v/v) in order to obtain a 100 mM solution. Make next dilutions (0.1 mM, 0.5 mM and 1 mM) in CM food, starting with lowest concentration and using the same final concentration of solvent for each treatment group. For the control vials use same volume of the solvent alone.
NOTE: it is recommended to keep the final concentration of the solvent as low as possible, bearing in mind that final concentration of ethanol should not exceed 2% in fly food. - Add the solution containing the right dilution of the selected EDC to the cornmeal-based food before solidification, mix thoroughly with a food processor, dispense 10 mL into vials, cover with cotton gauze and let dry at RT for 16 h before using.
NOTE: use this medium immediately after its preparation. - For the adult rearing, prepare different working EE2 solutions (10 mM, 50 mM and 100 mM, respectively) in 10% ethanol in water (v/v) and layer 100 µL of each onto the surface of the AM, in order to obtain the desired concentration of the EE2 (0.1 mM, 0.5 mM and 1 mM). For the control use same volume of the solvent alone.
NOTE: alternatively, add the solution containing the right dilution of the selected EDC to a small amount of AM in a 50 mL conical tube, vortex thoroughly and stratify 1 mL of it onto the surface of the AM vials.- Cover vials with cotton gauze, allow drying at RT for 12-16 h under gentle agitation and use them immediately.
NOTE: drying process should be adjusted experimentally because is depending on the ambient humidity.
- Cover vials with cotton gauze, allow drying at RT for 12-16 h under gentle agitation and use them immediately.
3. Rearing Flies
- Use a robust isogenic strain, such as Oregon R, maintained by several generation in the laboratory.
- Keep flies in a humidified, temperature-controlled incubator, with a natural 12 h light: 12 h dark photoperiod at 25 °C in vials containing CM food.
- In each assay, use vials at RT.
4. Lifespan Protocol
- Set up 20 vials of flies with 8 females and 4 males and house at 25 °C in CM (10 mL each).
- After 4 days discard flies and place vials back in the incubator.
NOTE: these flies can be used to start again to obtain other age-synchronized cohorts of the flies. - In the late afternoon of the day 9, remove all newly eclosed flies from the vials and return vials to the incubator.
NOTE: a few adults should begin to eclose as early as the ninth day; discarding these flies allows to collect a maximum number of synchronized flies, avoiding the careless selection of early emergent. - 16-24 h later, transfer the adult flies (1 day old) of both sexes into four groups of 250 mL bottles containing AM food supplemented with three different EDC concentrations and one with the solvent alone. If needed, collect another batch the next day.
- Maintain flies at 25 °C for 2-3 days to allow them to mate.
NOTE: the day of transfer to AM food vials corresponds to the first day of adulthood. - After two-three days, sort each cohort of flies by sex into two groups under light CO2 anesthetization. Randomly subdivide each sex into five vials per treatment at a density of 20 individuals per vial, until there are three replicates of 5 parallel vials for each gender per each treatment.
NOTE: Work with small groups of flies in order to prevent possible long-lasting health issues due to long exposure time to CO2. - Prepare a lifespan spreadsheet in which the number of dead flies is subtracted from the number of surviving flies to the previous transfer, in such a way as to automatically obtain the number of survivors at each transfer.
- Transfer flies into new vials containing the corresponding food every 3 days at the same time and check for death.
NOTE: the transfer must take place without anesthesia that could have a long-term negative effect on fly longevity.- At each transfer, record the age of the flies and the number of dead flies.
NOTE: the number of surviving flies is automatically calculated in the spreadsheet but it is recommended to check it visually. Flies that accidentally both escape or die during the transfer should not be considered. Be careful not to count twice dead flies carried to the new vial reporting this note in the spreadsheet. - Repeat steps 4.8 and 4.8.1 until all flies die.
- At each transfer, record the age of the flies and the number of dead flies.
- For each treatment group, create a survival curve as shown in Figure 1, in order to display the survival probability of a fly at any particular time.
- Perform three independent experiments for each treatment group of flies, by using 100 newly eclosed flies for each experiment.
- Prepare a table in which to report the mean lifespan (mean survival days of all flies for each group), the half death time (period of time in days required to reach 50% mortality) and the maximum lifespan (maximum amount of days needed to reach 90% mortality).
- Calculate the differences in percentage between each treatment group with respect to the control group.
- Perform statistical analysis to compare the different treatment groups.
Representative Results
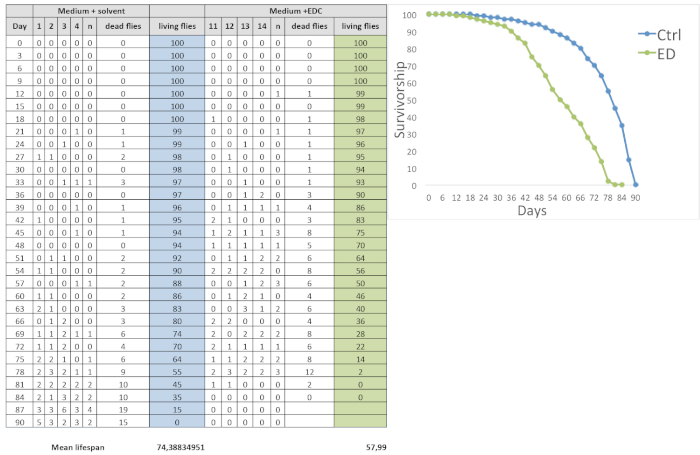
Figure 1: Lifespan curves. On the left, a representative table is reported in which the number of dead flies has been recorded every 3 days, both for the treatment group (medium + EDC [0.05 mM EE2]) and for the control group (medium + solvent), throughout the entire experimental period. The mean lifespan of each group has been calculated using the MATRIX SUM.PRODUCT; the treated group shortened the mean lifespan compared to the control group. On the right, a typical survival curve is shown of male flies fed with medium containing EE2 (0.05 mM) or only ethanol for the control. The survivorship curve decreased more rapidly for treated flies than for the control group, with an earlier turning drop point. Please click here to view a larger version of this figure.
Materials
| 17α-Ethinylestradiol | Sigma | E4876-1G | |
| Agar for Drosophila medium | BIOSIGMA | 789148 | |
| Cornmeal | CA' BIANCA | ||
| Drosophila Vials | BIOSIGMA | 789008 | 25 mm x 95 mm |
| Drosophila Vials | BIOSIGMA | 789009 | 29 mm x 95 mm |
| Drosophila Vials | Kaltek | 187 | 22 mm x 63 mm |
| Ethanol | FLUKA | 2860 | |
| Glass Bottle | 250 mL Bottles | ||
| Glass Vials | Microtech | ST 10024 | Flat bottom tube 100 x 24 |
| Instant Success yeast | ESKA | Powdered yeast | |
| Methyl4-hydroxybenzoate | SIGMA | H5501 | |
| Stereomicroscope with LED lights | Leica | S4E | |
| Sucrose | HIMEDIA | MB025 | |
| Hand blender Pimmy | Ariete | food processor |

