In Vitro Photoacoustic Flow Cytometry: A Non-invasive Flow Photoacoustic Technique to Detect Nanoparticle-bearing Circulating Ovarian Cancer Cells
Abstract
Source: Joel F. Lusk et al. Ovarian Cancer Detection Using Photoacoustic Flow Cytometry. J. Vis. Exp. (2020)
In this video, we present a photoacoustic flow cytometric detection of ovarian cancer cells bearing nanoparticles in a circulating in vitro flow chamber. This non-invasive technique successfully identifies circulating tumor cells in a flow system utilizing a contrast agent.
Protocol
Flow System Architecture
1. Flow chamber construction
- Using the provided Solid Works file, 3D print the flow tank using ABS thermoplastic or PLA plastic. Dimensions are provided below if SolidWorks is unavailable. Figure 1A shows a representation of this model. The body of the flow tank is 2.5 cm x 1.5 cm x 7.5 cm. The far ends of the flow tank include holes approximately 5 mm in diameter to allow for the entry of tubing containing the capillary tube.
NOTE: The flow tank has a 1 cm hole, perpendicular to the orientation of the capillary tube, for placement of the ultrasound transducer. A cylindrical extrusion with the same inner diameter as the hole extends 6 mm into the tank. For real-time imaging, the flow tank has a 1 mm x 3 mm slot directly below the capillary tube. - After printing the 3D flow tank, clean and assemble the system for use.
- Place glass coverslips over the 1 mm x 3 mm slot and the 1 cm hole in the flow system.
- Carefully seal with silicone to prevent leakage.
- Fit the capillary tube into the silicone cured tubes. Insert the tubes into the flow chamber through the side of the flow tank such that the glass capillary tube is directly above and in front of the 3 mm slot and the 1 cm hole.
- Seal the tubing using silicone to prevent leakage.
2. Photoacoustic flow system setup
NOTE: Figure 1B and Figure 1C show an example of the flow system architecture.
- Connect the transducer to an ultrasound pulser/receiver. Amplify the signal with a 59 dB gain.
- Connect the output of the filter to a multipurpose reconfigurable oscilloscope equipped with a built-in field-programmable gate array.
- Connect one of the tubes coming from the flow chamber to a T-junction, connected to two syringe pumps at each branch.
- Fill one of the syringe pumps with air and the other pump with the sample to be analyzed. Set the pump containing air to a flow rate of 40 µL/min and the pump having the sample to a flow rate of 20 µL/min. The resulting two-phase flow will produce sample volumes of 1 µL. At this flow rate, the system will test approximately 6.4 samples per minute.
NOTE: To maintain a consistent distribution of cells, lightly vortex each sample immediately before being tested. In addition, rotate the syringe every few minutes to prevent the cells from settling in the solution. - Connect the remaining tube exiting the flow system to a container with 10% bleach, to dispose of cells after they exit the flow system.
NOTE: Before utilizing the flow system, check for leaks, as these can affect the flow. Cells must be contained within a closed system to maintain biological safety during the procedure. - The design of the 3D printed tank allows for consistent and repeatable alignment between the transducer and laser light with minimal calibration. When placed correctly within the custom tank, the quartz capillary tube ensures that the transducer and laser are directly aligned.
- Place the section of the quartz capillary tube in direct alignment with the transducer, in the field of view of the microscope, allowing for careful placement of the optical fiber above the sample such that it illuminates the entire width of the tube.
- Irradiate the sample using an optical fiber channeling a diode-pumped solid-state laser operating at a wavelength of 1,053 nm. The laser light incident on the sample and the transducer used to measure the photoacoustic effect are both unfocused.
- The energy of the laser incident on the sample is approximately 8 mJ and the 10 Hz laser rate is sufficient to illuminate each sample multiple times as it passes through the system.
- Place the flow system on top of an inverted microscope and ensure both the laser pulse and the path of the sample are visible as the sample passes through the flow system. Record flow using a microscope-mounted camera.
- Record the ultrasound acquisitions utilizing data acquisition software. Trigger ultrasound and pulsed laser using the FPGA. Utilize PBS with 2% Tween, and FA-CuS NPs at a concentration of 100 µg/mL in PBS 2% Tween, as negative and positive controls, respectively.
- Utilizing a microscope-mounted camera, record both the firing of the laser and the passage of samples through the flow system. These recordings will be utilized to correlate the acoustic signal recorded by the transducer with the firing of the laser. As the samples pass in front of the firing of the laser, the signal can then be correlated to the resulting photoacoustic signal for analysis. At a sampling rate of 10 Hz, the laser will illuminate each plug several times.
Representative Results
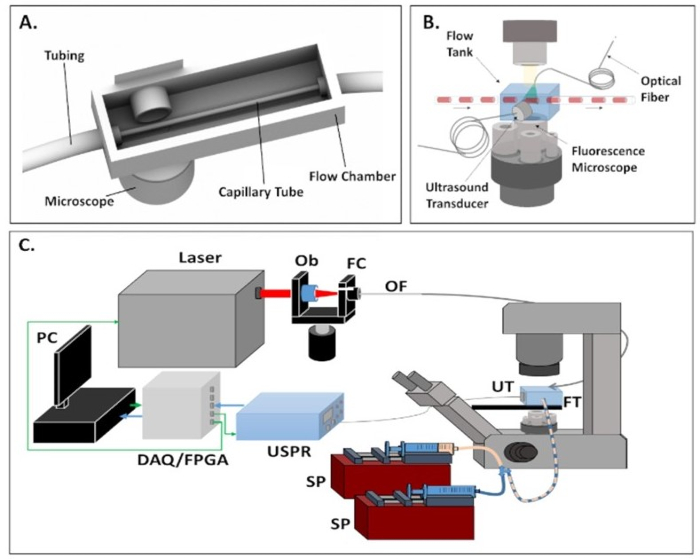
Figure 1. Representative images of photoacoustic flow cytometry system and flow chamber. (A) Detailed view of the 3D printed flow chamber. (B) Diagram of PAFC system. (C) Flow system architecture: SP = syringe pump; DAQ/FPGA = data acquisition/field programmable gate array; Ob = objective lens; OF = optical fiber; FC = fiber coupler; UT = ultrasound transducer; FT = flow tank.
Disclosures
The authors have nothing to disclose.
Materials
| 3D Printed Tank | Custom-made | ||
| Acquisition Card | National Instruments | PXIe-5170R | 250 MS/s, 8-Channel, 14-bit |
| Alconox | Sigma-Aldrich | 242985-1.8KG | Detergent used for cleaning glassware. |
| Amicon Ultra-15 Centrifugal Filters | Millipore | UFC903024 | |
| Amicon Ultra-4 Centrifugal Filters | Millipore | UFC803024 | |
| Data Acquisition software | National Instruments | NI LabVIEW 2017 (32-bit) | LabVIEW used to synchronize laser pulses with data acquisition. |
| Data Processing Software | Mathworks | Matlab R2016a | Reconstructions and graphs produced using Matlab software. |
| FBS | Sigma-Aldrich | F2442-500ML | |
| Pulsed Laser | RPMC Lasers Inc | Quantus-Q1D-1053 | Pulsed laser source with specifications 1053 nm, 8 ns pulse, 10 Hz maximum. |
| Pulser/Receiver | Olympus | 5077PR | Receives, filters, and amplifies photoacoustic signals. Operated with 59 dB Gain. |
| Quartz Capillary Tube | Sutter Instrument | QF150-75-10 | |
| Syringe Pumps | New Era Pump Systems Inc | DUAL-1000 | |
| Coupling Stage | Newport | F-91-C1-T | Stage for coupling pulsed light to objective. Holds FP-1A and LMH-10x-532 |
| Coupling Objective | Thorlabs | LMH-10x-532 | To couple pulsed light to optical fiber. |
| Norm-Ject 10 mL Syringes | HENKE SASS WOLF | 4100-X00V0 | |
| Transducer | Olynmpus | V214-BB-RM | Ultrasound detector with central frequency of 50 MHz and -6 dB fractional bandwidth of 82%. |
| Trypan Blue Solution 0.4% | Amresco | K940-100ML | |
| Optical Fiber | Thorlabs | FG550LEC | Used to expose sample to pulsed light. |
| Tween 20 | Sigma-Aldrich | P7949-100ML |

