Detrusor Smooth Muscle Cell Isolation: An Enzymatic Digestion Procedure to Obtain DSM Cells from Human Urinary Bladder Specimens
Abstract
Source: Malysz, J. et al. Preparation and Utilization of Freshly Isolated Human Detrusor Smooth Muscle Cells for Characterization of 9-Phenanthrol-Sensitive Cation Currents. J. Vis. Exp. (2020)
In this video, we demonstrate the isolation of detrusor smooth muscle cells from human urinary bladder specimens using a two-step sequential enzymatic treatment.
Protocol
All procedures involving human participants have been performed in compliance with the institutional, national, and international guidelines for human welfare and have been reviewed by the local institutional review board.
1. Dissection of DSM Tissues and Preparation of Mucosa-free DSM Pieces
- Examine the whole thickness urinary bladder specimen that arrived in the lab from the operating room in a tightly sealed container filled with the cold dissection/digestion solution (Figure 1 and Table 1 for composition of DS).
NOTE: The specimen is usually kept in cold DS from a few hours to overnight prior to arrival in the laboratory. For longer storage, DS (Table 1) is supplemented with 1 mM CaCl2. - Remove and rinse the human whole thickness DSM specimen (containing all layers including mucosa, DSM, and serosa) with ice-cold DS to wash out attached debris and blood.
- Pin the urinary bladder specimen, mucosa facing upward and serosa down, onto a silicone enantiomer-coated (Table of Materials) 150 mm diameter round dish filled with ice-cold DS (Figure 1B).
- Remove the adjacent adipose tissue, blood vessels, epithelium (urothelium) and muscularis mucosa from the specimen by sharp dissection using microscissors and forceps.
- Cut out several mucosa-free DSM pieces (~2–3 mm long and 4–6 mm wide) (Figure 1C).
2. Enzymatic Dissociation of DSM Pieces Yielding Freshly Isolated Single DSM Cells
- Place 3 to 6 DSM pieces into a tube containing 1 to 2 mL of pre-warmed (~37 °C) DS containing papain and dithiothreitol (DS-P, Table 1) and incubate DSM pieces in DS-P for 30–45 min at ~37 °C gently shaking the tube occasionally (once every 10–15 min).
NOTE: To optimally control the temperature for enzymatic treatment, tubes with tissue pieces and enzyme solutions are placed in either a glass tissue chamber filled with water connected to a circulating heated water bath (Figure 1D) or a high-precision temperature-controlled shaking water bath (Figure 1E). - Remove DS-P from the tube, briefly wash DSM pieces with ice-cold DS, discard cold DS from the tube leaving DSM pieces sitting at the bottom of the tube.
- Add 1 to 2 mL of DS-containing collagenase type II (DS-C, Table 1) to the tube with DSM pieces; gently mix and incubate for 25–40 min at ~37 °C gently shaking the tube occasionally (every 10–15 min).
- Discard DS-C and wash enzyme-treated DSM pieces 5–10 times with ice-cold DS.
- Following the last wash, leave DS solution inside the tube; gently triturate with a fire-polished Pasteur pipette several times to release single DSM cells.
- Place a few drops of DS solution containing dispersed DSM cells onto a glass-bottom chamber or a coverslip and visually inspect for the quality under a microscope (using a 20x or 40x objective) after at least 5 min following the application to allow the cells to adhere to the bottom.
- Immediately use freshly isolated DSM cells for electrophysiological experiments or store the cells in a tube containing DS at ~4 °C either on ice or in a refrigerator until use (typically for up to 8 h of preparation).
NOTE: Within the same preparation the quality of cells varies from highly viable to over-digested, dead DSM cells (Figure 2). When the sequential papain-collagenase method yields a very high number of unviable cells, the preparation is discarded, and a new digestion of DSM pieces is carried out but with reduced incubation intervals. If the procedure results in too few DSM cells, then for the subsequent digestion of DSM pieces, the incubation intervals are increased. Positive immunoreactivity to α-smooth muscle actin confirms the identity of DSM cells (Figure 3).
| Solution Type | Composition (in mM) |
| DS (Dissection/Digestion Solution) | 80 Na-glutamate, 55 NaCl, 6 KCl, 10 HEPES, 2 MgCl2, and 11 glucose, pH adjusted to 7.4 (with 10 M NaOH) |
| DS-P (Papain-containing DS) | DS containing 1-2 mg/ml papain, 1 mg/ml dithiothreitol and 1 mg/ml bovine serum albumin |
| DS-C (Collagenase-containing DS) | DS solution containing 1-2 mg/ml collagenase type II, 1 mg/ml bovine serum albumin, 0 or 1 mg/ml trypsin inhibitor and 100-200 μM Ca2+ |
| P (Pipette) | 110 CsOH, 110 aspartic acid, 10 NaCl, 1 MgCl2, 10 HEPES, 0.05 EGTA, and 30 CsCl,pH adjusted to 7.2 with CsOH, and supplemented with amphotericin-B (300-500 μg/ml) |
| E (Extracellular) | 10 tetraethylammonium chloride (TEA), 6 CsCl, 124 NaCl, 1 MgCl2, 2 CaCl2, 10 HEPES, and 10 glucose, pH adjusted to 7.3-7.4 with NaOH or CsOH, and 0.002-3 (2-3 mM) nifedipine |
Table 1: Compositions of dissection/digestion solution (DS), and pipette and extracellular solutions used in perforated patch-clamp experiments.
Representative Results
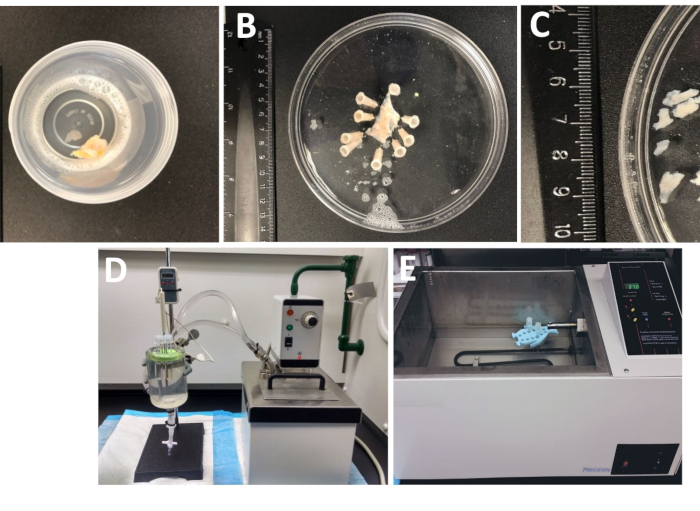
Figure 1: Summary of dissection steps resulting in the preparation of detrusor smooth muscle (DSM) pieces and setup used for enzymatic dissociation. Shown are images of: (A) a whole thickness human urinary bladder specimen provided from an open bladder surgery as extraneous surgical material in ice-cold DS, (B) the same preparation after pinning with a partially dissected DSM layer, (C) DSM pieces of variable dimensions cut out from the DSM layer ready for enzymatic digestion (smaller pieces) or other experimental investigations (larger pieces), (D, E) alternative setups used for enzymatic digestion of DSM pieces consisting of either (1) a temperature-controlled circulating water bath connected via tubing to a large glass tissue chamber filled with water, a rubber holder for tubes, plastic tubes containing DSM pieces and enzyme solutions prepared in dissection/digestion solution (DS, either DS-P or DS-C, Table 1) and a temperature probe linked to a display allowing for continuous monitoring (D), or (2) a large water-filled temperature-controlled bath containing a holder and tubes with DSM pieces and enzyme solutions €.
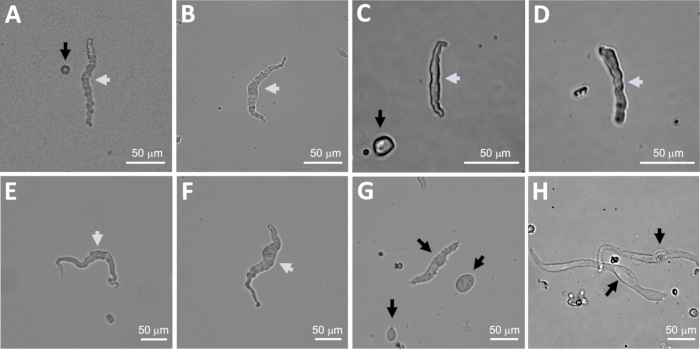
Figure 2: Representative bright-field images of human freshly isolated DSM cells obtained using the sequential papain-collagenase digestion method. (A–F) Displayed are images of viable, physiologically active DSM cells considered suitable candidates for attempting perforated patch-clamp recordings. (G, H) Images of non-viable or over-digested cells; such cells were avoided for patch-clamp experiments. White and black arrows in panels (A–H) point to DSM cells considered viable and non-viable, respectively, for attempting patch-clamp recordings. Note that the black arrows in panels (A, C, and G) point to cell fragments (circular pieces) or small cells lacking DSM morphology and in (H) the cells appear pale and dilated. Images are from three different urinary bladder specimens (A and B: patient-donor source one, C and D: patient-donor source two, and E–H: patient-donor source three).
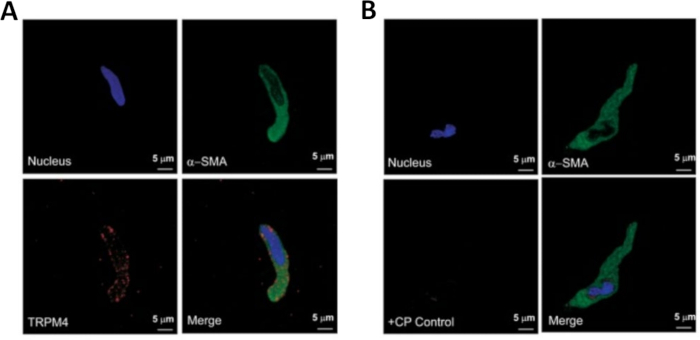
Figure 3: Expressions of transient receptor potential melastatin type 4 (TRPM4) channel and α-smooth muscle-specific actin immunoreactivities in single human DSM cells by immunocytochemistry analysis. (A) Shown are confocal images showing immunocytochemical detection of TRPM4 channel protein expression in a human DSM cell. Red staining (bottom left) indicates TRPM4 channel proteins; blue (DAPI) staining detects cell nuclei (top left); green staining indicates α-smooth muscle actin (α-SMA, top right); the merged image (bottom right) illustrates the overlap of all three images. (B) Confocal images illustrating attenuation of immunocytochemical detection of TRPM4 channel protein expression in the presence of a TRPM4-specific competing peptide (CP) in isolated human DSM cells. Blue (DAPI) staining indicates cell nuclei (top left); green staining is for α-smooth muscle actin (α-SMA, top right); the merged image (bottom right) illustrates the overlap of all three images. The results were verified in four separate experiments using DSM whole tissue or multiple DSM cells isolated from four patients. Images are used with permission.
Disclosures
The authors have nothing to disclose.
Materials
| 5 ml polystyrene round-bottom tube | Falcon | 352054 | Tubes for DS containing enzymes used in digestion steps |
| Analog vortex mixer | VWR | 58816-121 | |
| Bovine serum albumin | Sigma-Aldrich | A7906 | DS |
| Calcium chloride | Sigma-Aldrich | C1016 | Extracellular solution and DS |
| EGTA | Sigma-Aldrich | E3889 | Ca2+ chelator, used in intracellular pipette solution |
| Glucose | Sigma | G8270 | |
| Collagenase type 2 | Worthington Biochemical Corporation | LS004177 | DS-C |
| Cesium hydroxide hydrate | Sigma-Aldrich | C8518 | Intracellular pipette solution |
| CsCl | Sigma-Aldrich | 203025 | Extracellular and intracellular solutions |
| DL-Dithiothreitol (DDT) | Sigma-Aldrich | D9779 | Reducing agents used together with Papain |
| NaOH | Sigma-Aldrich | S8045 | |
| Nifedipine | Sigma-Aldrich | N7634 | L-type voltage-gated Ca2+ channel blocker |
| Floating foam tube rack/holder | VWR Scientific | 82017-634 | Used for holding tubes with enzymes for temperature control |
| Glutamic acid (Na salt) | Sigma-Aldrich | G1626 | DS |
| Papain | Worthington Biochemical Corporation | LS003126 | DS-P |
| MgCl2 (hexahydrate) | Sigma-Aldrich | M2670 | Extracellular and intracellular solutions |
| KCl | Fisher Scientific | BP366-1 | Extracellular solution |
| NaCl | Sigma-Aldrich | S7653 | Extracellular and intracellular solutions |
| Non-metalic syringe needle, MicroFil | WPI | MF-34G-5 | Filling of intracellular pipette solution |
| Pasteur pipette | FisherBrand | 13-678-20A | Tips are broken off and firepolished and used for titration of enzymatically treated tissues to release single DSM cells from pieces |
| HEPES | Sigma-Aldrich | H3375 | pH Buffer |
| Thermo Scientific Precision shaking water bath (model 2870) | Thermo Scientific | Discontinued | Water bath for temperature control of enzymatic digestion employed as an alternative to tissue chamber-circulating bath setup |
| Vinyl tubing | ColePalmer | 06405-3 | Multiple uses including for connecting tissue bath to circulating water bath |
| Water circulator bath, Haake D1 L | Haake | Discontinued | Connected to tissue bath |
| ZeissAxiovert 40C inverted microscope with 10x and 40x objectives | Carl-Zeiss | Discontinued | Part of patch-clamp rig setup |

