Continuous Drug Infusion System in Mouse Model: A Surgical Procedure to Implant Micro-osmotic Pump Infusion System in the Mouse Brain for Continuous Drug Delivery
Abstract
Source: Ajoy, R., Chou, S. Y. Studying the Hypothalamic Insulin Signal to Peripheral Glucose Intolerance with a Continuous Drug Infusion System into the Mouse Brain. J. Vis. Exp. (2018)
In this video, we demonstrate the assembly of a micro-osmotic pump brain infusion system followed by its surgical implantation in the mouse skull. This system enables continuous delivery of potential drugs and facilitates the study of their effects on the brain and brain tissue.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation of Micro-Osmotic Pump Infusion Systems
NOTE: Prepare the pump, artificial cerebrospinal fluid (aCSF) buffer, and drug (Met-CCL5/RANTES protein solution (10 ng/mL in aCSF)) under sterile conditions using buffers filtered with 0.2 µm filters and conduct all the procedures under the culture hood with gloves. The surgery procedures are conducted as follows:
- Prepare the micro-osmotic pumps one day before surgery: Fill the brain micro-osmotic pump with artificial cerebrospinal fluid (aCSF) with a 1 mL syringe and blunt-head needle provided along with the kit. Immerse the micro-osmotic pump in aCSF and place it on a shaker and gently shake overnight.
CAUTION: The pump should be filled with aCSF, and air bubbles should be avoided inside the pump (Figure 1A). - Before starting surgery, prepare the recombinant Met-CCL5/RANTES protein solution (10 ng/mL, diluted in aCSF) to be used in the experiment. Remove aCSF from the pump and fill the pump with the drug solution slowly until excess leaks out.
NOTE: 15 mL aCSF or Met-CCL5/RANTES solution is sufficient for 5-8 pumps.
CAUTION: Repeat the procedure to ensure that the pump is completely filled with the drug without bubbles inside. - Cut the catheter tubes into the desired length and attach them with the blunt-end brain infusion needle in the brain infusion kit. Fill the infusion kit and tubes with the drug.
- Finally, assemble and attach the brain infusion kit to the micro-osmotic pump.
CAUTION: No bubbles should be formed in the tube or the pump (Figure 1A). - Immerse the entire osmotic pump-brain fusion set in aCSF in a sterilized 50 mL tube to prevent the pump from drying out. The osmotic pump-brain fusion set is now ready to be used for surgery.
NOTE: The micro-osmotic pump systems can be used for long-term drug infusion. This ensures a safe and convenient mode of drug delivery into the mouse brain.
2. Intracerebral Ventricular Surgery – implantation of the micro osmotic pump
CAUTION: Sterilize the surgical environment with 75% ethanol and ensure that the people involved in the study are wearing sterile gloves and a clean lab coat. Surgical tools/ instruments must be autoclaved and dried before use, and subsequently sterilized with 75% ethanol in-between mice surgeries.
- Weigh the mouse and anesthetize it using intra-peritoneal injection (IP) with Ketamine/Xylazine (Ketamine 50 mg/kg, Xylazine 10 mg/kg).
CAUTION: Mice body weights lower than 24 g are not recommended for osmotic pump implantation surgery. - Mount and fix the mouse head onto the stereotactic apparatus (Figure 1B).
- Use a pair of surgical scissors and pincers to cut open the outer skin covering the skull. Use iodine to clean the peripheral skull.
- Separate the outermost layer of skin from the subcutaneous skin with the help of a pair of blunt-head pincers near the neck region for the osmotic pump-brain fusion set implantation (Figure 1C).
- Mark the infusion point with reference to the brain map (Figure 1D) using the stereotactic apparatus. In this experiment, the needle needs to be implanted in the 3rd ventricle region (Bregma: 0.0 mm lateral, 1.3 mm posterior, 5.7 mm ventral).
- Drill a hole using a nail drill around the area marked on the skull (Figure 1E).
CAUTION: Be careful not to break the mouse meninges and blood vessels, thus avoiding the disruption of micro-blood vessels in the brain. - Place the micro-osmotic pump-brain fusion set containing aCSF (as control) or drug (Met-CCL5/RANTES protein solution) under the skin behind the neck region and insert the brain infusion needle into the drilled hole to infuse the drug into the mouse brain (Figure 1E). The needle will penetrate the meninges and get into the ventricle. Fix the needle in place on the skull using surface desensitizing gel (Figure 1F) and wait 1-2 min until the glue dries. Next, cut off the projecting part on top of the needle (Figure 1G-H).
- Use a tissue adhesive glue to heal the operation wound on the head. Apply 50 µL of the glue on top of the wound, pull both sides of the skin together, and hold on for 30 s to allow the skin to seal (Figure 1I).
CAUTION: Use 100% alcohol pad to clean the wound after surgery and 100 ul penicillin with streptomycin to prevent infection. NOTE: Mouse skin will form scar tissue and heal in a few days following the administration of the surgical glue. The main advantage of the glue is the avoidance of surgical stitches which may cause skin irritation or inflammation. - Place the mouse in a clean cage kept on a warm plate (heated up to 37 °C) and wait until the mouse recovers from the anesthetic effect.
CAUTION: It is critical to maintain the mouse's body temperature to enhance the chance of survival after surgery. - After a one-week recovery period, the mice will be ready for further experiments, such as the Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT).
Representative Results
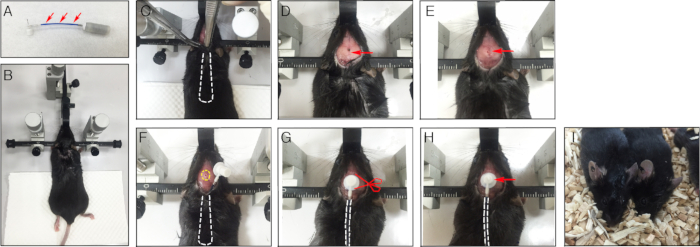
Figure 1. Osmotic pump preparation and implantation surgical procedure in mouse. (A) The brain infusion kit and pump preparation perfused with drug solution. Red arrows indicate the catheter tubes filled with liquid. (B) Fix and mount the mouse head onto the stereotactic apparatus. (C) Separate the outermost layer of the skin from the subcutaneous skin for the implantation of micro-osmotic pump-brain infusion set; dash lines indicate the location of osmotic pump implants. (D) The arrow indicates the infusion side. (E) Drill a hole around the marked area on the skull. (F) Place the osmotic pump-brain infusion set into the back of the mouse and insert the brain infusion needle into the drilled hole (dash circled). (G) Fix the needle onto the skull using tissue-adhesive glue and detach the top of the needle (Scissor pointed in G) as shown in (H). (I) Seal the wound using tissue adhesive glue.
Disclosures
The authors have nothing to disclose.
Materials
| Vetbond Tissue Adhesive | 3M | #1049SB | The glue used to seal the lesion site on the mouse head |
| LOCTITE 454 instant adhesive | Durect Corporation | #8670 | The glue used to fix the needle on the mouse skull |
| Alzet Micro- Osmotic Pump | Durect Corporation | #9922 | 0.11 μl per hour, 28 days |
| Brain infusion system | Durect Corporation | #8851 | 1-3 mm, used to perfuse the drug in to the mice brain |
| MIO NE116 CONTROL UNIT (nail drill) | Mio System | #E235-015 | To drill a hole in the skull of the mouse |
| aCSF formula | 119 mM NaCl 26.2 mM NaHCO3 2.5 mM KCl 1 mM NaH2PO4 1.3 mM MgCl2 10 mM glucose | Filter sterilize with a 0.22 μm filter apparatus, and store at 4°C. aCSF is stable for 3-4 weeks |

