Multisystem Monitoring of Epileptic Abnormalities in Rabbit: A Method for Simultaneous Video EEG, ECG, Capnography, and Oximetry Recording During Induced Seizures
Abstract
Source: Bosinski, C., et al. Multi-system Monitoring for Identification of Seizures, Arrhythmias and Apnea in Conscious Restrained Rabbits. J. Vis. Exp. (2021).
In this video, we demonstrate a multisystem monitoring method to evaluate neurological and cardiorespiratory abnormalities during intravenous injection of a seizure-inducing drug. The simultaneous monitoring of video EEG, ECG, oximetry, and capnography parameters helps to understand the real-time physiological changes associated with epileptic seizures.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. To enable the rabbits to acclimate in the restrainers and for the investigator to objectively confirm stabilization of cardiorespiratory rates, instrument all rabbits with the cardiorespiratory and neuronal sensors and perform continuous video monitoring for > 1 hour, 1 – 3 times per animal.
2. Intravenous medication experiment (Pentylenetetrazol, PTZ)
- To visualize the marginal ear vein, shave the posterior surface of the rabbit's ear. Use a 70% ethanol wipe to disinfect the site and dilate the marginal ear vein. This is indicated by the black dashed oval in Figure 1F.
- At this point, have one experimenter cover the rabbit's face with their hand to decrease the stress of the procedure to the rabbit. A second experimenter carefully cannulates the marginal ear vein with a sterile 25-G angiocatheter.
- Once the catheter is in the vein, place a sterile injection plug at the end of the catheter so that a needle can introduce medication intravenously. The location of the injection plug is indicated by a blue circle in Figure 1G.
- Make a splint by wrapping 4 x 4 inch gauze with tape so that it forms a tube shape and placing it inside the rabbit's ear. Then tape the splint to the ear so that the catheter is secured in place and remains upright, similar to the non-catheterized ear.
- Inject 1 mL of 10 USP units per mL of heparinized saline.
NOTE: The catheter and vessel should be visibly cleared of air and remain patent. If the catheter is not in the vessel, the syringe will not push easily and there will be accumulation of saline in the subcutaneous tissue. - Give rabbits incremental doses of PTZ intravenously from 1 mg/kg to 10 mg/kg in 1 mg/kg increments every 10 min. Make a note at the start of each dose to indicate which animal is being injected and the concentration of the medication.
NOTE: This enables assessments of the acute and additive effects of PTZ administration. Alternatively, to further assess the chronic effects of low dose PTZ, the rabbit is given repeated doses at each low dose concentration, 7 doses at 2 mg/kg, 3 doses at 5 mg/kg, then 3 doses at 10 mg/kg, each dose is separated by 10 min. - After each dose, carefully monitor the video-EEG-ECG-capnography-oximetry for any neuro-cardiac electrical and respiratory abnormalities and visual evidence of epileptiform activity. Note these changes in real-time and during post-analysis.
NOTE: Seizure activity often begins within 60 s of PTZ administration.
Representative Results
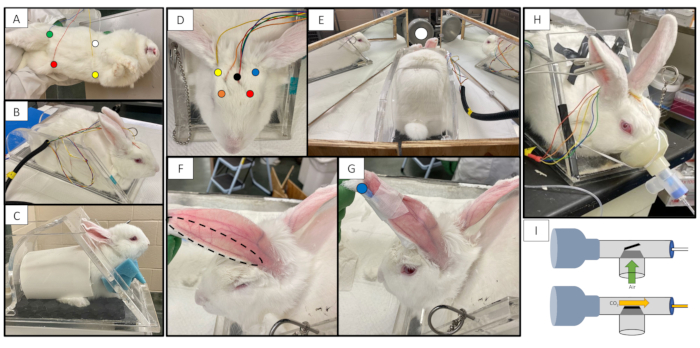
Figure 1: Rabbit connected to equipment. (A) Location of ECG electrodes, Left Arm is indicated by a yellow dot. Right Arm is indicated by a white dot. Left Leg is indicated by a red dot. Ground anterior to the right leg is indicated by a green dot. (B) Rabbit in restrainer with ECG and EEG electrodes attached. (C) Juvenile rabbit in a restrainer with appropriate modifications to accommodate a smaller rabbit, including a booster beneath the rabbit, neck foam and cut PVC pipe. (D) Rabbit in restrainer with location of EEG electrodes. Right Frontal is indicated by an orange dot. Left Frontal is indicated by a red dot. Right Occipital is indicated by a yellow dot. Left Occipital is indicated by a blue dot. The reference is indicated by a black dot. (E) Rabbit in restrainer with photic stimulator and mirror booth setup. Light source is indicated by a white dot. (F) Marginal ear vein after rabbit's ear has been shaven and wiped with alcohol. (G) Rabbit with angiocatheter securely taped in the left marginal ear vein. Site of injection plug is indicated with a blue dot. (H) Rabbit with facemask attached to the capnography tubing by a T-piece that contains a one-way valve. (I) Diagram of the facemask and T-piece connected to the capnography tubing. During inspiration, room air can enter the T-piece through a one-way valve (green arrow). During expiration, CO2 leaves the T-piece by entering the capnography tubing (yellow arrow.) Because of the small amount of dead space, very little CO2 is retained in the T-piece and is generally less than 5 mmHg.
Disclosures
The authors have nothing to disclose.
Materials
| 0.9% Sodium Chloride Irrigation, USP – Flexible Container | PFIZER (HOSPIRA) | 7983-09 | Dilutant |
| 10cc Luer Lock syringe with 20G x 1" Needle | Sur-Vet | SS-10L2025 | Used as a flush after drug injection |
| 4×4 gauze sponges | Fisher Scientific | 22-415-469 | Rolled in a tube to splint ear with angiocatheter |
| Computer | Dell | Optiplex 5040 | Acquisition computer |
| ECG Electrode | RhythmLink | RLSND116-2.5 | 13mm 35-degree bent (0.4 mm diameter) subdermal pin electrodes |
| EEG Electrode | RhythmLink | RLSP513 | 5-twist 13mm straight (0.4mm diameter) subdermal pin electrodes |
| EEGLAB (2020) | Swartz Center for Computational Neuroscience | Open Access | Can perform spectral analysis of EEG |
| Ethernet-to-ethernet adapter | Linksys | USB3G16 | Adapter for connecting the camera to the computer |
| Heparin Lock Flush | Medline | EMZ50051240 | To maintain patency of angiocatheter |
| IR Light | Bosch | EX12LED-3BD-8W | Facilitates recordings in the dark |
| LabChart Pro (2019, Version 8.1.16) | ADInstruments | N/A | ECG Analysis |
| JELCO PROTECTIV Safety I.V. Catheters, 25 gauge | Smiths Medical | 3060 | Used to catherize marginal ear vein |
| MATLAB (R2019b, Update 5) | MathWorks | N/A | Required to run EEGLAB |
| Microphone | Sony Stereo | ECM-D570P | Recording of audible manifestions of seizures |
| Micropore Medical Tape, Paper, White | 3M | 1530-1 | Used to secure wires and create ear splint |
| Natus NeuroWorks | Natus | LC101-8 | Acquisition and review software |
| Pentylenetetrazol (1 – 10 mg/kg always in 1mL volume) | Sigma-Aldrich | 88580 | Dilutions prepared in saline |
| Quantum Amplifier | Natus | 13926 | Amplifier / digitizer |
| Quantum HeadBox Amplifier | Natus | 22134 | 64-pin breakout box |
| Rabbit Restrainer | Plas-Labs | 501-TC | Various size rabbit restrainers are available. 6" x 18" x 6" in this study. |
| SpO2 ear clip | NONIN | 61000 | PureSAT/SpO2 |
| SpO2 sensor adapter | NONIN | 13931 | XPOD PureSAT/SpO2 |
| SRG-X120 1080p PTZ Camera with HDMI, IP & 3G-SDI Output | Sony | SRG-X120 | Impela Camera |
| Terumo Sur-Vet Tuberculin Syringe 1cc 25G X 5/8" Regular Luer | Sur-Vet | 13882 | Used to inject intravenous medications |
| Veterinary Injection Plug Luer Lock | Sur-Vet | SRIP2V | Injection plug for inserting the needle for intravenous medication |
| Webcol Alcohol Prep, Sterile, Large, 2-ply | Covidien | 5110 | To prepare ear vein before catheterization |

