Tartrate-Resistant Acid Phosphatase Staining: An In Vitro Technique to Detect TRAP Enzyme-Containing Cultured Osteoclasts
Abstract
Source: Dai, Q., et al. A RANKL-based Osteoclast Culture Assay of Mouse Bone Marrow to Investigate the Role of mTORC1 in Osteoclast Formation. J. Vis. Exp. (2018).
This video demonstrates the tartrate-resistant acid phosphatase staining technique to detect the presence of TRAP-containing granules inside osteoclasts. The technique forms magenta-colored dye granule deposits in the cytoplasm of osteoclasts, rendering them visible under an inverted microscope.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Preparation
- Generate osteoclast specific Raptor deletion mice (Raptorfl/fl; Ctsk-cre, hereafter RapCtsk) by mating Raptorfl/fl mice with Ctsk-cre mice. Use the Raptorfl/fl mice as the WT control in this study.
- Have a container with ice to keep isolated bone.
- Prepare culture medium.
- Prepare α-MEM culture medium by supplementing minimum essential medium alpha (α-MEM) with 1x glutamine, penicillin-streptomycin, and 10% fetal calf serum.
- Prepare bone marrow macrophage induction medium consisting of 50 mL of α-MEM medium and M-CSF at 20 ng/mL.
- Prepare osteoclast induction medium consisting of M-CSF and RANKL at 20 ng/mL, respectively, in 50 mL of α-MEM medium.
- Prepare the following buffers before TRAP staining.
- Prepare fix solution consisting of 6.5 mL of acetone, 2.5 mL of citrate solution and 0.8 mL of 37% (vol/vol) formaldehyde solution.
- Prepare TRAP staining solution.
- Prewarm deionized water to 37 °C.
- Add 100 µL of fast garnet GBC base solution and 100 µL of sodium nitrite solution into a 1.5-mL microtube and mix by gentle inversion for 30 s. Leave the mixture to stand for 2 min.
- Prewarm 9 mL of deionized water to 37 °C. Add 200 µL of diazotized fast garnet GBC base solution from step 1.4.2.2, 100 µL of naphthol AS-BI phosphate solution, 400 µL of acetate solution, and 200 µL of tartrate solution.
- Keep the TRAP staining mixture solution in a water bath at 37 °C.
2. Dissection (Day-1)
- Euthanize 3 one-month-old female WT and RapCtsk mice by CO2 separately. Perform CO2 inhalation with appropriate equipment by trained personnel.
- Immerse 6 mice in a beaker with 100 mL of 75% ethanol (vol/vol) for 5 min to prevent bacterial contamination and then place on a dissection board in a supine position. Make an incision at the distal of the tibia vertically and dissect the skin along the hind limb with ophthalmic scissors.
- Cut the articular ligament of the hip joint with scissors and dislocate the hind limbs from the trunk.
- Cut the articular ligament of the knee joint and carefully disassociate the tibia and femur from the knee joint. Gently remove the soft tissue with scissors.
- Place all bones of one genotype in of one mouse in each well of a six-well-plate with 2 mL of α-MEM on ice.
NOTE: To preserve cell viability, the bone should be kept in these conditions for less than 1 h.
3. Isolation (Day-1)
- Fill one well of a six-well plate with 75% ethanol (3 mL) and the other 5 wells with α-MEM medium (3 mL).
- Put all bones of one genotype in the ethanol wash for 15 s and wash bones 5 times with α-MEM medium for 10 s each time.
- Cut off the epiphyses with the scissor and insert a 0.45-mm syringe needle into the bone cavity and flush marrow out with α-MEM medium into a 50-mL centrifuge tube.
- Flush the bone cavity using the same method from the other end of the bone. Repeat at least twice to wash the bone cavity thoroughly until the bone is pale.
- Centrifuge the bone marrow to obtain a cell pellet at 800 x g and 4 °C for 5 min. Aspirate the supernatant.
- Add 3 mL of red blood cell lysis buffer into the centrifuge tube and pipette gently to resuspend the bone marrow. Keep the centrifuge tube on ice for 8 min to lyse red blood cells.
- Add 6 mL of α-MEM medium into the centrifuge tube to stop cell lysis. Centrifuge at 500 x g and 4 °C for 10 min and aspirate the supernatant.
- Resuspend in 3 mL of α-MEM culture medium and place the cells into a six-well plate (generally one mouse per well).
- Incubate at 37 °C in a 5% CO2 incubator overnight.
4. Plating (Day 0)
- Transfer the supernatant to a 15 mL centrifuge tube to collect the unattached cells the next morning.
- Centrifuge at 800 x g and 4 °C for 5 min and aspirate the supernatant.
- Resuspend in 4 mL of α-MEM culture medium.
- Take 20 µL of the cell solution and mix with 20 µL of Trypan Blue. Add 10 µL of the mixture to the counting chamber and obtain the cell count per mL of solution.
- Add an appropriate volume of α-MEM culture medium to obtain a cell solution of 500,000 cells/mL.
- Add 500 µL and 50 µL of bone marrow macrophage induction medium into each well of a 24-well plate and 96-well plate, respectively.
- Add 500 µL and 50 µL of cell solution into each well of a 24-well plate and 96-well plate, respectively.
- Incubate at 37 °C in a 5% CO2 incubator for 3 days.
5. Differentiation (Days 3-7)
- Three days after plating, collect 50 µL medium from each well of the 96-well-plate and freeze at -20 °C, and then aspirate the residual medium.
- Add 1 mL and 100 µL osteoclast induction medium into each well of a 24-well plate and a 96-well plate, respectively.
- Incubate at 37 °C for 2 days.
- Collect 50 µL of medium from each well and aspirate the residual medium.
- Add osteoclast induction medium into each well.
- Incubate at 37 °C for 1 day.
NOTE: At this time point, large, multinucleated osteoclasts should be seen under an inverted microscope (Figure 1A). - If osteoclasts are not seen, change the osteoclast induction medium and observe daily until osteoclasts are seen.
- Collect 50 µL of medium from each well of the 96-well plate after osteoclasts have formed.
6. Tartrate Resistant Acid Phosphatase (TRAP) Staining
NOTE: Mature osteoclasts may be present after 6-7 days of in vitro culture (step 4).
- Aspirate medium and wash gently 3 times with 1x PBS.
- Fix the cells in each well of the 96-well plate with 100 µL of fix solution for 30 s at room temperature.
- After fixation, aspirate the fix solution and wash gently 3 times with deionized water prewarmed to 37 °C. Aspirate deionized water.
- Add 100 µL of TRAP stain into each well of the 96-well plate and place the plate in an incubator at 37 °C for 30 min and shield from light.
- After staining, aspirate the TRAP stain and gently wash 3 times with deionized water.
- Image osteoclasts using an inverted microscope at 40X magnification.
Representative Results
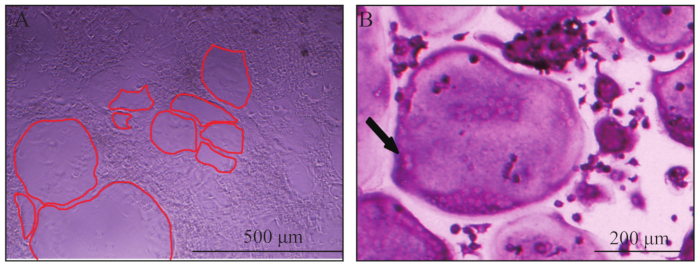
Figure 1: Representative view of osteoclasts. (A) Giant osteoclasts observed in bright field on day 6. The red line outlined the border of osteoclasts. (B) A typically giant, multinucleated, TRAP positive (wine red) osteoclast. The black arrow indicated two nuclei of the multinucleated osteoclast.
Disclosures
The authors have nothing to disclose.
Materials
| Raptorfl/fl mice | The Jackson Laboratory | 13188 | |
| Ctsk-cre mice | a gift from S. Kato, University of Tokyo, Tokyo, Japan | ||
| α-MEM | Corning | 10-022-CVR | |
| Glutamine | Gibico | 25030081 | |
| Penicillin streptomycin | Gibico | 15140122 | |
| Fetal calf serum | BioInd | 04-001-1A | |
| Recombinant mouse M-CSF protein | R&D | Q3U4F9 | |
| Recombinant mouse RANKL protein | R&D | Q3TWY5 | |
| RBC lysis buffer | Beyotime | C3702 | |
| Trypan blue | Sigma-Aldrich | 302643 | |
| Acetone | Shanghai Chemical Co. Ltd. | ||
| Citrate solution | Sigma-Aldrich | 915 | |
| Formaldehyde solution | Shanghai Chemical Co. Ltd. | ||
| Acid Phosphatase, Leukocyte (TRAP) Kit | Sigma-Aldrich | 387A-1KT | |
| Fast Garnet GBC Base solution | Sigma-Aldrich | 3872 | |
| Sodium Nitrite Solution | Sigma-Aldrich | 914 | |
| Naphthol AS-BI Phosphate Solution | Sigma-Aldrich | 3871 | |
| Acetate solution | Sigma-Aldrich | 3863 | |
| Tartrate solution | Sigma-Aldrich | 3873 | |
| Dulbecco's phosphate-buffered saline | Corning | 21-031-CVR | |
| 37% formaldehyde | Xilong scientific | ||
| IX71 | Olympus | ||
| 0.45-mm Syringe | |||
| Scissor | |||
| Mosquito forcep |

