An Assay to Study the Impact of Photodynamic Treatment with a Photosensitizer on Macrophage Metabolic Activity
Abstract
The video demonstrates a technique for evaluating the influence of photodynamic treatment (PDT) with a photosensitizer on the metabolic activity of murine macrophages. The cells undergo treatment with the photosensitizer followed by exposure to UV light, initiating PDT. This process triggers the photosensitizer to generate reactive oxygen species (ROS) within the cells, causing cellular toxicity. The impact of ROS is then assessed by enzymatic antioxidants through a colorimetric assay, providing insights into the metabolic activity within the cells.
Protocol
1. Safety considerations
- Personnel should use personal protective equipment (PPE).
- Handle the macrophages in a Class II Biological safety cabinet.
- Avoid self-exposure to UV light.
2. Preparation of macrophages
- Cultivate a murine macrophage cell line, RAW 264.7, on a tissue flask containing 10 mL of RPMI-1640 medium, supplemented with 20 mg/mL streptomycin, 2 mM L-glutamine, 20 U/mL penicillin and 10% fetal bovine serum (FBS).
- Place the flask in a 5% CO2 incubator set at 37˚C until 80% confluence is achieved.
- Gently scrape off the cells using a cell scraper.
- Transfer the suspended cells into a 15 mL centrifuge tube.
- Use the trypan blue stain to determine cell viability.
NOTE: A viability score that is above 85% is preferred. - Use a hemocytometer to adjust the cell concentration of macrophages to 1 × 106 cells/mL using a 10 mL solution of fresh, sterile RPMI-1640 medium.
- Dispense 100 µL suspension of cells into wells of a sterile 96-well flat-bottom microtiter plate.
- Incubate the plate overnight in a 5% CO2 incubator at 37˚C.
NOTE: Before use, the overnight spent media should be aspirated and replaced with 100 µL of fresh, sterile RPMI-1640 media.
3. Compound preparation
- Dissolve the photosensitizer, PQ, in distilled water to prepare a stock solution.
- Serially dilute the stock solution further in RPMI-1640 media to yield a PQ concentration gradient – at twice the desired final concentration.
NOTE: The final volume of distilled water in RPMI-1640 media should never exceed 1%. - Draw a 100 µL solution of the prepared PQ and dispense it into appropriate wells that contain the already seeded macrophages.
4. Implementation of PDT
- See Table 1 for the sorting of macrophages. Here, have designated microtiter plates for: 1) ultraviolet light (UVL)-only macrophages (not exposed to PQ), 2) PQ-only macrophages (exposed to dark light (DL)), and 3) PDT-only macrophages.
- To initiate the experiment (after 30 min), macrophages seeded in the following plates should be exposed to:
- UVL-only plate; UVL for 8 min,
- PQ-only plate; DL for 8 min, and
- PDT-only plate; UVL for 8 min.
NOTE: In the current work, we determined that PQ has a UV/Vis absorption of 260 nm, which is in the UV range of the germicidal lamp. Due to the latter, we used a germicidal ultraviolet C (UVC) lamp fitted in a Class II Biological safety cabinet as a light source to implement PDT. The lamp has a nominal power of 30 Watts. In the current study, the cells were kept at approximately 20 cm from the lamp. At this distance, the lamp is estimated to have a radiation output of 625 µW/cm2.
5. Macrophage metabolic activity assay
- After completing the PDT as indicated above, initiate the tetrazolium salt reaction by adding 50 µL of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) to the same wells with irradiated macrophages.
NOTE: The above tetrazolium reaction should be carried out on all macrophages as sorted per Table 1. - Add 4 µL of menadione.
- Incubate the plate at 37˚C in 5 % CO2 for 3 h.
- Measure product formation by reading the absorbance of the well contents at 492 nm.
Table 1. An illustration of the experimental conditions to be set up before initiating the experiment. In this protocol, PQ was tested at 0, 6, 60 and 600 µM.
| Macrophages seeded in the UVL-only plate | Macrophages seeded in the PQ-only plate | Macrophages seeded in the PDT-only plate |
| Macrophages with 0 µM of PQ | Macrophages with 0 µM of PQ | – |
| – | Macrophages with 6 µM of PQ | Macrophages with 6 µM of PQ |
| – | Macrophages with 60 µM of PQ | Macrophages with 60 µM of PQ |
| – | Macrophages with 600 µM of PQ | Macrophages with 600 µM of PQ |
NOTE: All the seeded plates should be allowed to stand for 30 min under ambient light and temperature. During this period, cells in the 1) UVL-only plate will react to ambient light, 2) PQ-only plate will react to different PQ concentrations, and 3) PDT-only plate will, likewise, react to different PQ concentrations under ambient light.
Representative Results
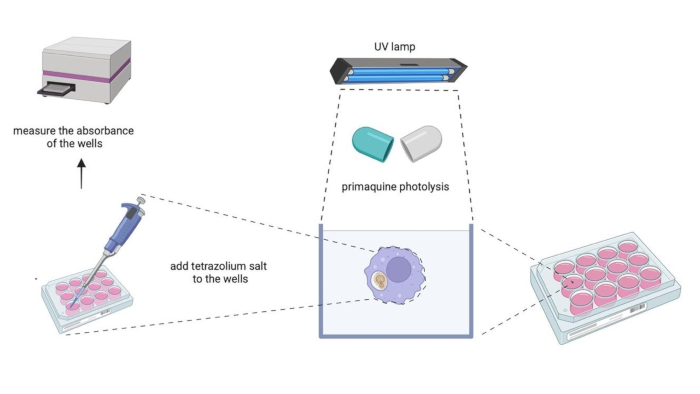
Figure 1 is a schematic representation that shows the macrophage metabolic activity protocol.
Disclosures
The authors have nothing to disclose.
Materials
| Primaquine | Merck | Used as a photosensitizer | |
| Phosphate buffer solution (PBS) | Sigma-Aldrich | Wash and dilute cells | |
| 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) | Invitrogen | Used to measure the metabolic activity of the macrophages | |
| Menadione | Sigma-Aldrich | Used in conjunction with XTT | |
| 50 mL centrifuge tube | Lasec | Used to contain macrophages | |
| Haemocytometer | Marienfield | To determine the cell concentration of the macrophages | |
| RPMI- 1640 medium | Biochrom | Cultivation media for macrophages | |
| Fetal bovine serum (FBS) | Biochrom | Used to supplement the RPMI-1640 medium | |
| 5% CO2 incubator | Thermo-Fisher | Used to incubate the macrophages | |
| Cell scrapper | Lasec | Used to detach macrophages from the tissue flask | |
| Trypan blue stain | Sigma-Aldrich | To enumerate live and dead cells | |
| Penicillin/Streptomycin/ L-glutamine | Sigma-Aldrich | Added to the RPMI-1640 medium | |
| 96-well flat-bottom microtiter plate | Greiner Bio-One | Used as a vessel within which reactions were carried-out | |
| 1.5 mL plastic tubes | Merck | Used as a vessel within which reactions were carried-out | |
| UVL | ESCO | Used as a light source to photosensitise PQ | |
| EZ Read 800 spectrophotometer | Biochrom | To read the optical density | |
| Fluoroskan Ascent FL microplate reader | Thermo Scientific | To measure fluorescence |

