Isolation of Gingival Immune Cell Network from Murine Teeth Blocks
Abstract
Source: Dutzan, N. et al., Isolation, Characterization and Functional Examination of the Gingival Immune Cell Network. J. Vis. Exp. (2016)
This video demonstrates the isolation procedure of the gingival immune cell network from a mouse model. The enzymatic digestion releases the gingival tissue from the attached teeth and bone, which is further mechanically digested, releasing immune cells from the gingiva. The isolated cells are ready for immunological experiments.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Prepare in Advance
- Prepare Complete media: RPMI supplemented with 2 mM L-glutamine, 100 units/ml penicillin, 100 ug/ml streptomycin, and 10% FBS.
- Prepare DNase media: 50 ml of RPMI media supplemented with 7.5 µg DNase (Make fresh, keep on ice at all times).
- Prepare Collagenase-DNase media: 5 ml of DNase media plus 3.2 mg/ml of Collagenase Type IV (Make fresh, keep on ice at all times).
- Pre-cool centrifuge to 4 °C.
- Warm shaker incubator to 37 °C.
2. Isolation, Dissection, and Cell Isolation from Gingival Blocks
- Euthanize mice by exposing them to CO2 in an appropriate chamber, according to Animal Research Advisory Committee (ARAC) guidelines.
- Open the thoracic and abdominal cavity to expose the heart and internal organs. To perfuse, make an incision in the inferior vena cava and perfuse 3 ml of PBS via the left ventricle using a 3 ml syringe with a 27 G needle.
- Immobilize the body and head of the mouse on a pad with the stomach facing up.
- Cut each commissure of the lip towards the neck with scissors, separating the skin that covers the mandible and neck region, and exposing the underlying tissues and muscles. Dissect the lower lip to uncover the mandible.
- Cut between the lower incisors to separate both sides of the mandible and immobilize each side to access the oral cavity.
- Visualize and dissect the palate away from the nasal cavity.
- Dissect areas using a 10-blade and include maxillary and mandibular molar areas with surrounding gingiva (Figure 1). Make vertical incisions just anterior to the first molar and posterior to the third molar and a horizontal incision at the border of the gingiva (white collar of tissue surrounding teeth).
- Place the maxillary and mandibular blocks in a 50 ml conical tube with 5 ml Collagenase/DNase (keep on ice).
- Incubate in a shaker incubator at 37°C for 1 hr and during the last 5 min of incubation add 50 µl of 0.5 M EDTA.
- Add 5 ml of cold DNase media to the 50 ml tube with tissues and gently swirl to mix.
- Transfer the 4 blocks of tissue to a petri dish and cover them with 500 µl of DNase media. Remove gingiva from each block of tissue, using a scalpel blade and pointing the blade into the gingival and interdental areas.
- Transfer the tissues and the media from the petri dish as well as the previous DNase media used during dissection to a new 50 ml tube through the cell strainer (70 µm size). Wash with DNase media (3-5 ml) all of the instruments used and filter.
- Using the plunger of a sterile 3 ml syringe, mash the tissue against the strainer, filtering with cold DNase media (30 to 35 ml).
- Wash the cell strainer with cold DNase media.
- Centrifuge at 4 °C, 314 x g for 6 min.
- Discard the supernatant, and re-suspend it in 1 ml of complete media.
- Count cells (the expected amount should range between 8 x 105 to 1.2 x 106 cells with a viability of >80%) using an automated cell counter.
Representative Results
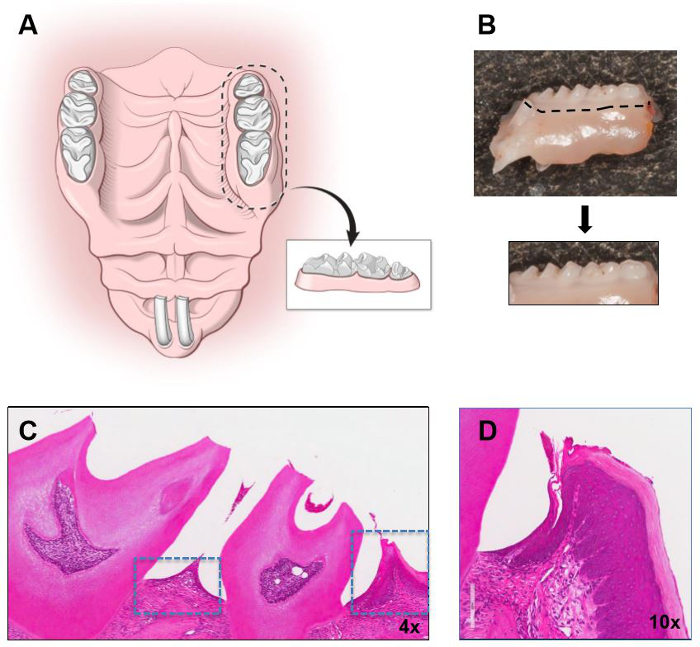
Figure 1: Isolation of murine gingiva. Illustration demonstrating the borders of dissection in the maxilla and the isolated gingival segment to be used for gingival tissue dissection. (B) Segment of the murine maxilla (molar area) with an outline for block dissection, and isolated block. Hematoxylin and Eosin stain (H&E) of a maxillary molar segment with gingival tissues outlined is shown in low (C) and higher magnification in (D). Original magnifications indicated.
Disclosures
The authors have nothing to disclose.
Materials
| Fine Scissors | Fine science tools | 14058-11 | |
| Scalpel Handle #3 | Fine science tools | 10003-12 | |
| Scalpel Blades #10 | Fine science tools | 10010-00 | Sterile |
| Splinter Forceps | Integra Miltex | 6-304 | |
| Needles with regular bevel | BD Medical | 305109 | 27G, 12.7 mm length |
| Monoject syringes | Covidien | 8881513934 | Luer-lock tip, 3mL |
| PBS, pH 7.4 | Life Technologies | 10010-049 | Without Calcium and Magnesium |
| RPMI 1640 | Lonza | 12-167F | Without L-glutamine |
| DNase I from bovine pancreas | Sigma-Aldrich | DN25-1G | |
| Collagenase type IV | Gibco (by Life technologies) | 17104-019 | |
| Fetal Bovine Serum | Gemini Bio-products | 100-106 | |
| Gentamicin 50 mg/ml | Quality biological | 120-098-661EA | |
| Pen Strep | Gibco (by Life technologies) | 15140-122 | |
| L-Glutamine | Gibco (by Life technologies) | 25030-081 | |
| 0.5M EDTA pH 8.0 | Quality biological | 351-027-721EA | |
| 50 mL tubes | Corning | 352070 | Polypropylene, sterile |
| 70 μM Cell Strainers | Corning | 352350 | |
| Petri dishes | Corning | 351029 | Sterile |

