Malassezia-Induced Skin Inflammation and Fungal Burden Analysis in a Murine Model
Abstract
Source: Sparber, F. et al., Infecting Mice with Malassezia spp. to Study the Fungus-Host Interaction. J. Vis. Exp. (2019)
This video demonstrates Malassezia-induced skin infection in mice. Malassezia oil suspension is applied over the wounded ear, triggering inflammation and swelling of the ear tissue. Later, the ears are dissected, homogenized, and spread on an agar plate for growth. The number of colonies indicates the fungal burden resulting from skin inflammation.
Protocol
All procedures involving animal models have been reviewed by the local institutional animal care committee and the JoVE veterinary review board.
1. Cultivation of Malassezia under laboratory conditions
NOTE: Store all the reagents and media used for this protocol at room temperature (RT, 20 – 25 °C) unless stated otherwise, as the lower temperature can inhibit fungal growth.
- Prepare the liquid-modified Dixon (mDixon) medium for Malassezia growth. To prepare 500 mL of liquid mDixon medium, dissolve 18 g Malt extract, 10 g desiccated Ox-bile, 5 mL Tween-40, 3 g Peptone, 1 mL Glycerol, and 1 mL Oleic Acid in 500 mL distilled H2O (dH2O). Adjust the medium to pH 6 with HCl and autoclave. Store the medium at RT.
- Prepare mDixon agar plates by adding 7.5 g agar to 500 mL mDixon medium prior to autoclaving. Slowly cool down the mDixon agar after autoclaving using a steering bar and a magnetic heating plate to avoid partial solidification of the medium while cooling down.
- Once the agar has cooled down to 50 – 60 °C, dispense the liquid into Petri dishes in a laminar flow hood and let them dry at RT overnight.
NOTE: The agar plates can be stored at 4 °C for several weeks when wrapped and kept upside down to avoid evaporation. - Obtain Malassezia isolates and revive lyophilized stocks of Malassezia according to the instructions obtained by the provider.
- Inoculate 10 mL of liquid mDixon medium in a sterile 100 mL Erlenmeyer flask with the revived Malassezia suspension according to the instructions obtained by the provider. Incubate the culture in a shaking incubator at 30 °C and 180 rpm.
- Inspect the growth of the Malassezia culture regularly by checking for the appearance of cream color and turbidity. Growth kinetics depend on the species and strain of Malassezia and may be particularly slow when Malassezia is freshly revived from a lyophilized stock. (Figure 1A).
- Prepare glycerol stocks by mixing 3 parts of the densely grown Malassezia culture in mDixon medium with 1 part of sterile 99% glycerol. Aliquot the Malassezia/glycerol mixture into sterile screw-cap tubes and store at -80 °C.
- For in vitro propagation, plate Malassezia from the liquid culture in mDixon or from the frozen glycerol stock onto a mDixon agar plate (brought to RT from 4 °C) using an inoculation loop.
NOTE: Transfer mDixon agar plates to RT from 4 °C prior to the use, since cold mDixon agar inhibits fungal growth. - Incubate the agar plate(s) with Malassezia upside down in aN (non-shaking) incubator at 30 °C. Inspect the growth of Malassezia colonies regularly.
NOTE: Malassezia colonies on mDixon agar plates appear within 3 – 5 days and are cream-colored dull, and smooth with convex elevation (Figure 1B). - Store Malassezia colonies on mDixon agar plates at RT for ~ 2 weeks. Thereafter, prepare a new mDixon agar plate by streaking Malassezia from the frozen glycerol stock as described in 1.7.
2. Preparation of the inoculum for experimental Malassezia infection of mice
- Inoculate 10 mL of liquid mDixon medium in a sterile 100 mL Erlenmeyer flask with 3 – 5 individual Malassezia colonies from a mDixon agar plate (see step 1, Figure 1B).
- Incubate the Malassezia culture for ~ 48 to 96 h at 30 °C and 180 rpm until the culture is cream-colored and turbid (Figure 1A).
NOTE: The time necessary for Malassezia growth depends on the Malassezia species and strain and the amount of fungus used for inoculation. - Transfer 2 mL of the Malassezia culture into a sterile 2 mL microcentrifuge tube and centrifuge for 1 min at 10,000 x g.
- Discard the supernatant and wash the pellet by suspending it in 1 mL of phosphate-buffered salt solution (PBS). Centrifuge again for 1 min at 10,000 x g.
- After the washing, suspend the pellet in 1 mL of PBS by vigorous pipetting and measure the optical density of the solution at 600 nm (ODA600) using a spectrometer. Dilute the Malassezia suspension 20 – 50 x with PBS for the OD measurement to ensure that the reading is between 0.1 and 1.
NOTE: The density of a 3-day culture of Malassezia generally varies between 15 and 30 ODA600, depending on the Malassezia species and strain and on the number of yeast cells used for inoculation of the culture (step 2.1). Malassezia tends to form aggregates, therefore, vigorous pipetting is necessary to ensure the homogeneity of the suspension. - Aliquot a volume of the Malassezia suspension in PBS that corresponds to a density of 4 ODA600 into a sterile 2 mL tube. Prepare 1 tube per animal to be infected.
- Centrifuge the tubes containing Malassezia for 1 min at 10, 000 x g.
- Discard the supernatant and suspend the Malassezia pellet in 200 µL of native olive oil (corresponding to 2 ODA600 yeast cells/100 µl olive oil).
NOTE: Olive oil was found to be a good vehicle for epicutaneous infection with Malassezia, as Malassezia is a lipophilic and lipid-dependent yeast. Olive oil is better absorbed by the skin than PBS. However, be aware that it is not easy to suspend Malassezia in olive oil. Improve the Malassezia/olive oil suspension by vortexing. Keep the suspension at RT until it is used for infection. - Prepare tubes with olive oil alone for mock infection of control animals.
3. Infecting mice with Malassezia
- Order female C57BL/6 mice at an age of 6 – 8 weeks and allow them to acclimatize in the experimental animal facility for at least one week. Calculate for 3 – 5 mice per group, including an uninfected control group.
- Prepare a sterile anesthetic cocktail containing 1.3 mg/mL Xylazine and 6.5 mg/mL Ketamine in PBS. 5 mL of the anesthetic cocktail is enough to anesthetize 20 animals. Adjust the volume of the cocktail according to the number of animals to be anesthetized.
- Anesthetize animals by injecting 10 µL/g bodyweight of anesthetic cocktail intraperitoneally (corresponding to 65 mg Ketamine and 13 mg Xylazine per kg body weight) and place the anesthetized animals onto a heating pad at 37 °C.
NOTE: At the indicated dose, animals usually remain anesthetized for ~ 30 – 60 min.
- Anesthetize animals by injecting 10 µL/g bodyweight of anesthetic cocktail intraperitoneally (corresponding to 65 mg Ketamine and 13 mg Xylazine per kg body weight) and place the anesthetized animals onto a heating pad at 37 °C.
- Check the reflexes by pinching the rear foot with forceps to ensure that the animals are fully anesthetized.
- Apply an eye cream to the eyes to prevent dehydration during anesthesia.
- Optionally, measure the ear thickness of both ears using a caliper (0 – 5 mm range). Measure two different areas of each ear and calculate the average ear thickness per ear.
NOTE: Measuring ear thickness is optional and depends on the research question. However, if the ear thickness is used as a readout for skin inflammation, it is necessary to measure the baseline ear thickness prior to infection. (see Step 4). - Optionally, disrupt the epidermal barrier of the dorsal ear skin by mild tape stripping: manually apply a small piece of tape to the skin and remove it again. Repeat for 5 consecutive rounds using a fresh piece of tape for each round.
NOTE: Malassezia induces a more pronounced skin inflammation in barrier-disrupted skin compared to unperturbed skin (Figure 2A). - Topically apply 100 µL (2 ODA600) of the Malassezia/olive oil suspension onto the dorsal side of each ear using a sterile pipette. Include a control group of animals that are treated with olive oil only (vehicle-treated control group).
NOTE: Vortex the Malassezia/olive oil suspension vigorously to ensure a homogenous Malassezia suspension immediately prior to the application. - Leave the anesthetized animals on the heating pad to avoid hypothermia until they show signs of recovery (whisker movement, increased breathing rate, etc.).
- Inject 200 µL of sterile and pre-warmed 2% glucose solution subcutaneously into the nuchal fold to support their metabolism and rehydration.
NOTE: To prepare a sterile 2% glucose solution, dissolve 1 mg glucose in 50 mL PBS and filter it using a 0.2 µm filter. The solution can be stored at 4 °C. - Transfer the animals back to their cage.
4. Analysis of Malassezia-induced skin inflammation
NOTE: This procedure describes the analysis of Malassezia-induced ear swelling during infection which serves as a parameter of skin inflammation. A prerequisite for analyzing the fungus-induced ear swelling is to measure the baseline ear thickness prior to tape-stripping and/or infection (Step 3.5).
- Prepare isoflurane chamber for short-term anesthesia of Malassezia-infected and control animals.
- Transfer one animal at a time to the chamber and wait for the animal to be fully anesthetized.
NOTE: Signs of proper anesthesia include complete body relaxation as well as slow and heavy (flank) breathing. Carefully monitor anesthesia as extended exposure to isoflurane can be fatal. - Remove the animal from the chamber and place it onto a tissue.
- Measure the thickness of the ear(s) using a caliper (range 0 – 5 mm). Measure two different areas of each ear and calculate the average thickness per ear (see step 3.5).
- Transfer the animal back to the cage.
NOTE: Isoflurane anesthesia is very short-lived, and the animals recover within ~ 30 s after removal from the isoflurane chamber. - Calculate the increase in ear thickness by subtracting the average baseline ear thickness, measured prior to the tape stripping and/or infection, from the average ear thickness measured at each time point after infection.
- Plot the calculated values as the increase in ear thickness or, alternatively, as the total ear thickness over time for each animal or group of animals (Figure 2B).
5. Analysis of fungal burden in the infected skin
- Prepare a sterile 2 mL microcentrifuge tube for each ear to be harvested, containing 0.5 mL of sterile 0.05% nonidet P40 (NP40) in dH2O and an autoclaved steel ball (5 mm diameter).
- Weigh the tubes using a precision balance and write down the precise weight.
- Euthanize the mice by CO2 asphyxiation.
- Remove the ear(s) at the base and transfer into the tube containing 0.5 mL of sterile 0.05% NP40 in dH2O, as described in steps 5.1 – 5.2.
- Weigh the tube containing the ear tissue and calculate the actual weight of each sample by subtracting the weight of the tube without the organ from the weight of the tube with the organ.
- Homogenize the ear tissue for 6 min at 25 Hz using a tissue homogenizer. Ensure that the tissue is well homogenized.
- Plate 100 µL of each sample (corresponding to 1/5 of each homogenate, dilution factor = 5) onto mDixon agar plates and incubate the plates upside down in a 30°C incubator.
NOTE: The amount of homogenate plated should be adjusted according to the fungal load to be expected. Make sure to plate sufficient homogenate to obtain at least 10 and no more than 250 colonies per plate to allow easy enumeration. Optionally, plate multiple plates per sample with different dilutions of homogenate. - Inspect the growth of Malassezia colonies regularly.
NOTE: Colonies usually become visible after 2 – 3 days. The time necessary for Malassezia colonies to grow depends on the species and strain of Malassezia. - Count the colonies per plate.
- Calculate the number of colony-forming units, CFU/g tissue by using the following formula:
CFU/g tissue = (number of colonies/plate) x (dilution factor) / (weight of the skin sample in g).
NOTE: The approximate minimal detection limit can be assessed using the following formula: minimal detection limit = (1 colony/plate) x (dilution factor) / (average weight of all skin samples in g). - Fungal loads are usually plotted on a logarithmic scale (Figure 2C).
Representative Results
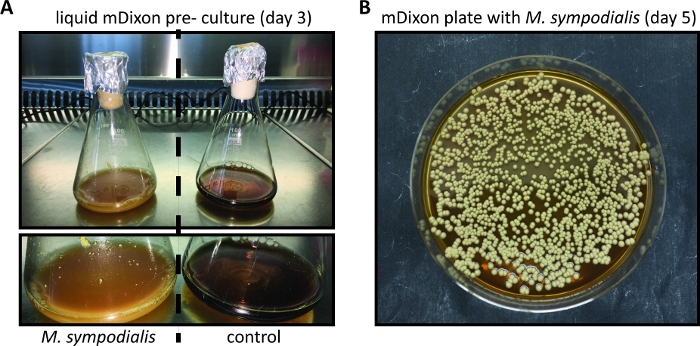
Figure 1: In vitro cultivation of Malassezia. (A) M. sympodialis strain ATCC 42132 grown for 3 days at 30 °C and 180 rpm in liquid mDixon medium (left) next to a control Erlenmeyer flask containing mDixon medium that was not inoculated (right). (B) Colonies of M. sympodialis strain ATCC 42132 on mDixon agar after 5 days of incubation at 30 °C.
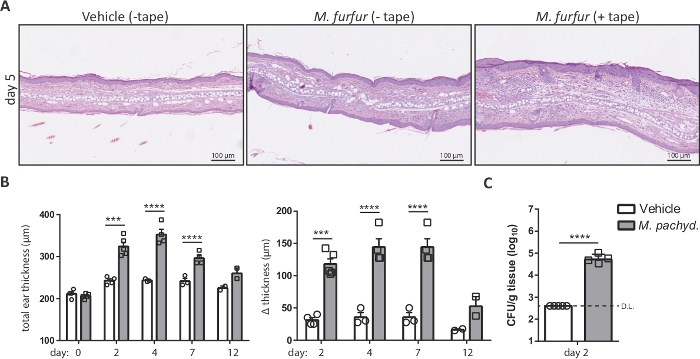
Figure 2: Analysis of Malassezia skin infection on the basis of ear thickness and fungal burden. (A) Histology of ear sections obtained from C57BL/6 mice that were treated with olive oil (vehicle, left) or infected with M. furfur strain JPLK23 for 5 days (middle and right). On the right, the ear skin was tape-stripped prior to infection. Sections were stained with hematoxylin and eosin (H&E). (B) Summary graphs showing the increase in ear thickness over time for C57BL/6 mice that were exposed to Malassezia or left uninfected as controls. The absolute thickness of M. pachydermatis strain ATCC 14522-exposed or vehicle-treated ear skin at each time point is displayed on the left; the increase in ear thickness at the indicated time points relative to the baseline on day 0 is shown on the right. (C) Fungal burden in the skin of C57BL/6 mice that were infected with M. pachydermatis strain ATCC 14522 or treated with olive oil as a control (vehicle). In both cases, the skin was tape-stripped. Each symbol in the summary graphs B and C represents one animal. The statistical significance of the differences between groups was calculated using one-way ANOVA (B) or Student's t-test (C). ***p <0.001, ****p <0.0001, D.L.: Detection Limit
Disclosures
The authors have nothing to disclose.
Materials
| Agar | Sigma-Aldrich | A1296-1KG | |
| Attane Isoflurane | Piramal Healthcare | – | |
| Biosaftey cabinet (BSC) Faster Ultra Safe | DASIT GROUP | TEC 5594 | BSL2 certified |
| Centrifuge | Eppendorf | 5415D | Compatible with 2ml Eppendorf tubes |
| Dessicated Ox-bile | Sigma-Aldrich | 70168-100G | |
| Eppendorf Tubes (2 ml) | Eppendorf | 0030 120.094 | |
| Glucose | Sigma-Aldrich | 49159-5KG | |
| Gylcerol (99 %) | Honeywell | 10314830 | |
| Heating pad | Eickenmeyer | 648048 | |
| Incubator Hereaus B20 | Heraeus | 412047753 | BSL2 certified |
| Ketasol (100 mg) | Graeub AG | 6680416 | |
| Magentic heating plate MR Hei-Standard | Heidolph Instruments | 442-1355 | |
| Malassezia spp. | ATCC | 14522, 14521, 42132 | |
| Malt extract | Sigma-Aldrich | 70167-500G | |
| Multiply Biosphere Tubes (200 µl) | Sarstedt AG | 7084211 | Safelock |
| Native olive oil | – | – | Commerc. available |
| Nonidet P40 | Axon Lab | A1694,0250 | |
| Oditest measurment devise | Kroeplin | S0247 | Range 0-5 mm |
| Oleic Acid | Sigma-Aldrich | 75090-5ML | |
| Peptone | Oxoid | LP0037 | |
| Petri dishes | Sarstedt AG | 82.1473 | |
| Phosphat buffered salt solution (PBS, 1x) | Amimed/Bioconcept | 3-05F39 | |
| Rompun (2 %) | Bayer | KP0BFHR | |
| Shaking incubator Infors Minitron | Infors | – | BSL2 certified |
| Spectrometer | Jenway | 20308 | Optical density measurement at 600nm |
| Spectrometer Cuvettes | Greiner Bio-One | 613101 | |
| Stainless Steel balls (5mm) | ABF | KU.5G80 1.3541 | |
| Syringes 1 ml Sub-Q | BD Bioscience | 305501 | |
| Tissue Lyzer II | Quiagen | 85300 | |
| Transpore Hypoallergic Tape | 3M | 1527-1 | |
| Tween 40 | Sigma-Aldrich | P1504-100ML | |
| Vitamin A Retinoli Palmitas Eye Cream | BAUSCH & LOMB | Commerc. available |

