Organotypic Slice Culture of GFP-expressing Mouse Embryos for Real-time Imaging of Peripheral Nerve Outgrowth
Summary
We present a method to prepare organotypic slices of mid-gestation mouse embryos for the cultivation and time-lapse imaging of peripheral nerve outgrowth.
Abstract
For many purposes, the cultivation of mouse embryos ex vivo as organotypic slices is desirable. For example, we employ a transgenic mouse line (tauGFP) in which the enhanced version of the green fluorescent protein (EGFP) is exclusively expressed in all neurons of the developing central and peripheral nervous system1, allowing the possibility to both film the innervation of the forelimb and to manipulate this process with pharmacological and genetic techniques2. The most critical parameter in the successful cultivation of such slice cultures is the method by which the slices are prepared. After extensive testing of a variety of methods, we have found that a vibratome is the best possible device to slice the embryos such that they routinely result in a culture that demonstrates viability over a period of several days, and most importantly, develops in an age-specific manner. For mid-gestation embryos, this includes the normal outgrowth of spinal nerves from the spinal cord and the dorsal root ganglia to their targets in the periphery and the proper determination of skeletal and muscle tissue.
In this work, we present a method for processing whole embryos of embryonic day (E) E10 to E12 into 300 – 400 micrometer slices for cultivation in a standard tissue culture incubator, which can be studied for up to two days after slice preparation. Critical for the success of this approach is the use of a vibratome to slice each agarose-embedded embryo. This is followed by the cultivation of the slices upon Millicell culture membrane inserts placed upon a small volume of medium, resulting in an interface culture technique. One litter with an average of 7 embryos routinely produces at least 14 slices (2-3 slices of the forelimb region per embryo), which varies slightly due to the age of the embryos as well as to the thickness of the slices. About 80% of the cultured slices show nerve outgrowth, which can be measured througout the culturing period2. Representative results using the tauGFP mouse line are demonstrated.
Protocol
Part 1: Preparing for slicing and culturing.
- Prepare 10-cm tissue culture plates with slicing medium (DMEM, 25% 1x HBSS, 25% fetal bovine serum, 0.5% glucose, 1 mM glutamine, 2.5 mM HEPES, pH 7.3) and 3-cm Millicell-CM 0.4-μm culture membrane inserts and keep in incubator at 37°C and 5% CO2.
- Heat up 4% low melting point agarose in PBS in the microwave and keep it on heating plate so that it stays at approximately 37°C.
- Fill 10-cm bacteriological Petri dishes with PBS (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8mM KH2PO4) and place on ice.
- Set up microtome cooling device or ensure that buffer tray and cooling elements are stored in the freezer to pre-cool it.
Part 2: Embryo embedding.
- Dissect embryos from the uterus and examine them with an inverted fluorescent microscope to check for GFP expression.
- Place embryos on an inverted 10-cm Petri dish and orient them using Whatman paper strips to remove excessive PBS.
- Apply agarose on embryo to fix it in this position. Let agarose solidify.
- Limit the area surrounding the embryo by cutting with a razor blade.
- Rotate the embedded embryo onto its other side.
- Apply additional agarose on embryonic tissue to ensure that the embryo is completely embedded.
- Prepare agarose block with clean edges and mount on vibratome chuck using Loctite 406, a special glue similar to “Krazy Glue”.
Part 3: Slicing procedure.
- Set up the precooled buffer tray and the cooling element.
- Insert the chuck with the glued tissue and add 1x HBSS (Ca2+-Mg2+-free HBSS, 10 mM HEPES buffer pH 7.3, 500 U/ml penicillin/streptomycin) until covered.
- Insert and fix a precleaned (70% Ethanol) microtome blade.
- Prepare 350 – 450 μm slices and transfer them using shortened glass pasteur pipettes into tissue culture plates kept on ice.
- Using a pair of forceps, carefully remove agarose from each slice and transfer to Millicell culture membranes. About 4 slices can be cultured on one membrane in a 10-cm tissue culture plate filled with 6 mL of culture medium.
- Incubate slices at 37°C and 5% CO2 (Culture medium: DMEM, 25% 1x HBSS, 25% fetal bovine serum, 0.5% glucose, 1mM glutamine, 2.5 mM HEPES, pH 7.3). If one performs time-lapse imaging series one should keep the volume of medium constant. In the case of timed imaging series for a short time frame it is sufficient to leave the medium as it is. For longer culture periods changes after 12-20 hours are recommended.
Part 4: Imaging spinal nerve outgrowth at the microscope.
- Image spinal nerves placing a 10-cm tissue culture plate, containing Millicell culture membranes with slices, under an upright fluorescent microscope.
- Label the orientation of Millicell culture membranes on microscope stage to position correctly during the next imaging time point.
- Image spinal nerve outgrowth using 4x (numerical aperture [NA] 0.1), 10x (NA 0.3), or 20x (NA 0.5) objectives.
Figure 1 shows an imaging series depicting the spinal nerve outgrowth during 20 hours of culture with 4x and 10x objectives.
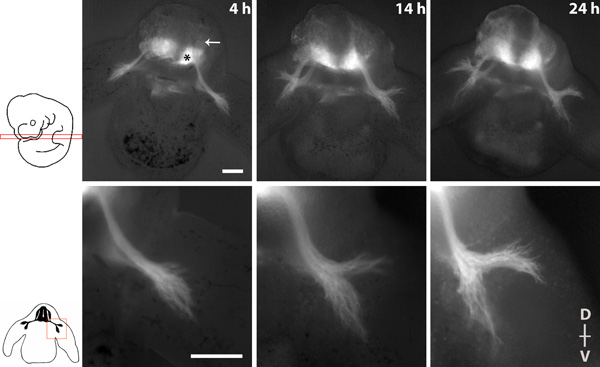
Figure 1 Imaging series of spinal nerve outgrowth in a transverse slice of a homozygous tauGPF embryo. * = motor neurons of the ventral spinal cord, arrow = DRG. The dorsal (D)-ventral (V) axis of the slice is indicated. Scalebars: 200 μm.
Discussion
In an extensive comparison of methods to prepare embryonic slice cultures of mid-gestation mouse embryos (E10 – E12), we have observed that a vibratome produces without question the most reliable results with respect to both the overall viability of the cultures and the reproducibility of the nerve outgrowth patterns. In contrast, slices prepared using a McIlwain tissue chopper3 proved to be completely inviable. We originally employed a guillotine method4, in which an entire litter of embryos could be simultaneously prepared by slicing with tungsten wires wrapped serially around a cutting grate to produce 400-μm sections1. Although this technique had the advantage of speed of preparation, it yielded at most one viable section per embryo and showed a large amount of variability in outgrowth parameters. For these reasons, we feel that a vibratome-based method is the superior choice despite the longer time in preparation. Although this solution by necessity requires a vibratome, the results are vastly superior to what has been achieved through other “low-tech” approaches, and thus justifies the investment.
Acknowledgements
The authors acknowledge the original source for the idea to perform slice culture upon mouse embryos5. We would like to acknowledge Joachim Kirsch for generous scientific support and Anna Degen for acting as our gofer during the filming. This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft: Sonderforschungsbereich 488, Teilprojekt B7/B9) and the University of Heidelberg (Excellence Cluster Cellular Networks).
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| HBSS 10x | GIBCO | 14180 | ||
| Dissection tools | FST | various | ||
| L.M.P. agarose | Invitrogen | 15517-022 | ||
| Whatmann paper | Whatman Int. | 3030917 | ||
| Shortened firepolished pipettes | ||||
| DMEM | GIBCO | 41966 | ||
| FBS | GIBCO | 10270-106 | ||
| Pen Strep | GIBCO | 15140 | ||
| L-glutamine 100x | GIBCO | 25030 | ||
| Vibratome | Microm International GmbH | HM 650 V | ||
| Fluorescent microscope | Olympus | BX61WI | ||
| analySIS | Soft Imaging System | |||
| Millicell-CM inserts | Millipore | PICMORG 50 | ||
| 10 cm culture plates | Greiner Bio-One | 633171 | ||
| LOCTITE 406 | Henkel | 142580 | ||
| Razor blades | Thermo Fisher | none | ||
| Dissecting microscope | Nikon | SMZ800 | ||
| HEPES | Roth | 9105.2 | ||
| Glucose | Sigma | G7021 | ||
| x4 objective | Olympus | PL series | ||
| x10 objective | Olympus | UPLFL –PH series | ||
| Filter | Olympus | U-MNIBA2 | ||
| CCD camera | Soft Imaging System | SIS F-View II | ||
| Equipment for heated chamber | Leica | CTI-Controller 3700 and incubator S #11531171 |
References
- Tucker, K. L., Meyer, M., Barde, Y. A. Neurotrophins are required for nerve growth during development. Nat Neurosci. 4, 29-37 (2001).
- Brachmann, I., Jakubick, V. C., Shaked, M., Unsicker, K., Tucker, K. L. A simple slice culture system for the imaging of nerve development in embryonic mouse. Dev Dyn. 236, 3514-3523 (2007).
- Collingridge, G. L. The brain slice preparation: a tribute to the pioneer Henry McIlwain. Journal of neuroscience methods. 59, 5-9 (1995).
- Katz, L. C. Local circuitry of identified projection neurons in cat visual cortex brain slices. J Neurosci. 7, 1223-1249 (1987).
- Hotary, K. B., Landmesser, L. T., Tosney, K. W. Embryo slices. Methods Cell Biol. 51, 109-124 (1996).

