Coronary Artery Ligation and Intramyocardial Injection in a Murine Model of Infarction
Summary
Numerous genetic manipulations and/or intramyocardial injections of genes, proteins, cells, and/or biomaterials are superimposed upon the dimension of time in studies of acute ischemia/ reperfusion injury and chronic remodeling in mice. This video illustrates the microsurgical procedures for ischemia/reperfusion, permanent coronary artery ligation, and intramyocardial injection studies.
Abstract
Mouse models are a valuable tool for studying acute injury and chronic remodeling of the myocardium in vivo. With the advent of genetic modifications to the whole organism or the myocardium and an array of biological and/or synthetic materials, there is great potential for any combination of these to assuage the extent of acute ischemic injury and impede the onset of heart failure pursuant to myocardial remodeling.
Here we present the methods and materials used to reliably perform this microsurgery and the modifications involved for temporary (with reperfusion) or permanent coronary artery occlusion studies as well as intramyocardial injections. The effects on the heart that can be seen during the procedure and at the termination of the experiment in addition to histological evaluation will verify efficacy.
Briefly, surgical preparation involves anesthetizing the mice, removing the fur on the chest, and then disinfecting the surgical area. Intratracheal intubation is achieved by transesophageal illumination using a fiber optic light. The tubing is then connected to a ventilator. An incision made on the chest exposes the pectoral muscles which will be cut to view the ribs. For ischemia/reperfusion studies, a 1 cm piece of PE tubing placed over the heart is used to tie the ligature to so that occlusion/reperfusion can be customized. For intramyocardial injections, a Hamilton syringe with sterile 30gauge beveled needle is used. When the myocardial manipulations are complete, the rib cage, the pectoral muscles, and the skin are closed sequentially. Line block analgesia is effected by 0.25% marcaine in sterile saline which is applied to muscle layer prior to closure of the skin. The mice are given a subcutaneous injection of saline and placed in a warming chamber until they are sternally recumbent. They are then returned to the vivarium and housed under standard conditions until the time of tissue collection. At the time of sacrifice, the mice are anesthetized, the heart is arrested in diastole with KCl or BDM, rinsed with saline, and immersed in fixative. Subsequently, routine procedures for processing, embedding, sectioning, and histological staining are performed.
Nonsurgical intubation of a mouse and the microsurgical manipulations described make this a technically challenging model to learn and achieve reproducibility. These procedures, combined with the difficulty in performing consistent manipulations of the ligature for timed occlusion(s) and reperfusion or intramyocardial injections, can also affect the survival rate so optimization and consistency are critical.
Protocol
- Sterile surgical instruments (Table 1) and 3″ cotton tipped applicators are placed on a sterile underpad. The bead sterilizer (Germinator 500) is turned on.
- Mice (age: > 6 weeks; wt: > 18g) are anesthetized with an i.p. injection of 20μl/g BW of tribromoethanol (250mg/kg; duration – approximately 40 minutes).
- When the mice are unresponsive to toe-pinch, a sterile lubricant (Tears Renewed) is applied to the eyes to protect them from desiccation and the left side of the chest is coated with depilatory (e.g. Nair) to remove fur from the skin.
- The depilatory is washed away with warm running water and betadine/alcohol swabbing is used to disinfect the surgical area.
- The mouse is placed on a warm deltaphase isothermal pad which is fixed to a plexiglass table. Each limb is immobilized using tape and a thick thread is placed horizontally under the top teeth to hold the upper jaw in place.
- The table is positioned vertically and a fiberoptic light is shone directly onto the neck region for transesophageal illumination. This requires precise placement such that the opening of the throat is viewed as a well lit orifice, thus enabling the trachea to be visualized to facilitate insertion of the PE tubing.
- The tubing is then connected to the ventilator (connected to a 95% O2/5% CO2) to administer constant positive pressure ventilation (TOPO ventilator; rate 125 breaths/min; peak inspiratory pressure 10-12 cmH2O; *note: settings vary with strain and gender 1-3). Once ventilation is confirmed by synchronous chest movements, the connection is fixed to the pad with tape to avoid extubation during the surgery.
- Using toothed forceps to pull skin up and away from the chest, a #10 sterile scalpel blade attached to a #3 scalpel handle is used to make a 1.5cm incision in the skin parallel to the sternum.
- Curved Vanna microscissors are used to cut the pectoralis muscles and make a small hole in the intercostal muscle.
- Straight, blunt microscissors are used to cut through 3 ribs.
- A 9mm pediatric ophthalmic speculum is used to retract the rib cage.
- Using the curved forceps, pull the pericardium away from the heart and use the toothed forceps to gently tear it open.
- Using the Castroviejo needle holder, a 6mm tapered point 3/8 needle threads the 8-0 polyethylene suture underneath the left anterior descending coronary artery (along the long axis of the heart) perpendicular to it.
- For a temporary ligature that can be removed for timed reperfusion, a sterile 0.5-1cm piece of PE90 is placed on the heart in parallel to the coronary artery. The suture, which has first been looped under the coronary artery, is then tied to the tubing. At the time it is to be released, the ligature is loosened. This can be repeated as desired and the time of occlusion/reocclusion can be modified 4. Depending on the length of the protocol and the type of anesthesia used, supplementation may be necessary.
- For a permanent occlusion, the ligature laced under the coronary artery is simply tied. Blanching and dyskinesia are apparent and the long end of the suture is cut 5-10.
- For intramyocardial injection(s), a sterile Hamilton syringe with a 30 gauge sterile beveled needle is introduced into the base of the heart above the area of injury on the right side of the ligature. The needle is then advanced into the area of injury and withdrawn slightly so that the bevel can be seen approximately at the border zone. Some of the solution in the syringe (2-3μl) is injected into the heart and the needle is held in place. The syringe is withdrawn another 1-3mm and the rest of the solution is injected. The syringe is held in place until the bleb that is formed by the solution dissipates. The needle is then removed. If there is any bleeding, a cotton-tipped applicator is gently pressed onto the needle insertion site until the bleeding stops 5-7.
- Once the myocardial manipulations are complete, the rib retractors are removed and the thoracic cavity is closed with 2-3 mattress sutures using 6-0 surgipro suture.
- Two-three mattress sutures are then made to close the pectoralis muscles, 1-3 drops of 0.25% marcaine 1:10 in sterile saline (0.1ml/25g mouse) is applied to the muscle and then 2-3 mattress sutures are made to close the skin.
- The mouse is removed from the ventilator. Once rhythmic, rapid, shallow breathing is verified, the mouse can be extubated.
- 0.5ml warm sterile saline is injected into the dorsal subcutaneous space and the mouse is placed on a warming pad in a cage until it regains mobility (1 hour minimum).
- For survival experiments, mice are placed back into their cages and returned to the vivarium until the time of sacrifice. During first 2 days, moistened food is placed on the cage floor to facilitate feeding (so they don’t have to reach up which may cause pain) and buprenorphine should be administered every 6-12hr. Post-operative care also includes daily monitoring for the first week to verify adequate mobility, grooming, and eating habits.
- The surgical instruments are wiped clean with ethanol and inserted into the bead sterilizer before the next surgery.
- At the time of sacrifice, mice are anesthetized with sodium pentobarbital (65mg/ml; 55-65 mg/kg). When an adequate plane of anesthesia is achieved, the thoracic cavity is opened.
- While the heart is still beating, a syringe with a 23 gauge needle containing cold potassium chloride (KCl, 30mM) or 2,3-butanedione monoxime (BDM; 10mM) is used to puncture the posterior basal region of the ventricle and the solution is slowly injected into the chamber until the heart is arrested in diastole.
- Once the heart is removed, a syringe containing PBS is used to retrogradely perfuse rinse the heart to remove any blood that remains. For acute studies, at the end of the reperfusion period, the LAD is re-ligated at the original point of occlusion. A solution containing 1% Evan’s blue is injected into the aorta. Once the heart is extracted, it is cut transversely into 3 sections of equal thickness, incubated in 1% 2,3,5-triphenyltetrazolium chloride, and imaged for morphometric analyses 11. For chronic studies, the heart is then immersed in fixative, then processed and embedded according to routine procedures. Slides can then be stained histologically and imaged for morphometric analyses (using Scion, NIH Image J, or Image Pro Plus) 9,10,12.
Representative Results:
When done correctly, the survival rates in mice (male: age 8-10 weeks, 22-28g; female: age 10-12 weeks, 20-26g) are: over 90% in acute ischemia/reperfusion and ischemic preconditioning experiments, over 85% in permanent artery ligation studies, and approximately 80% for intramyocardial injections. Since early injury is more readily visible by metabolic changes rather than structural, infarct size determination in ischemia/reperfusion and ischemic preconditioning experiments is performed by infusing 1% Evan’s blue dye into the aorta which will perfuse the heart that is not supplied by the LAD (Figure 1A). Once the heart is removed and transversely cut in half, the tissue is incubated in 1% solution of 2,3,5-triphenyltetrazolium chloride to measure infarct size (Figure 1B). The areas are measured using Scion or NIH imaging software which can be calibrated using a micrometer imaged at the same magnification. These numbers are used to calculate area at risk/left ventricle and infarct size/area at risk 11. Strain differences can result in variations in body weight and heart size and so care should be taken to normalize these measures to heart weight, body weight, or tibia length for comparative purposes.
Permanent artery ligation results in gross structural changes such as necrosis, wall thinning, and chamber dilation. Comparison of the effects of treatment and/or time on infarct size and necrosis relative to the left ventricle, chamber area, septal wall and left ventricular free wall thickness in the permanent occlusion model (Figure 2A) can also be measured using Scion or NIH imaging software. Collagen staining with picrosirius red/fast green (Figure 2B) can be used to measure insterstitial fibrosis which correlates to functional indices of wall stiffening8-10. The image in Figure 3 represents the distribution of 6ul solution (Evan’s blue) injected into the border zone of the heart following permanent artery ligation. Notice that it proceeds in the direction of the injury as well as toward the base and also transmurally.
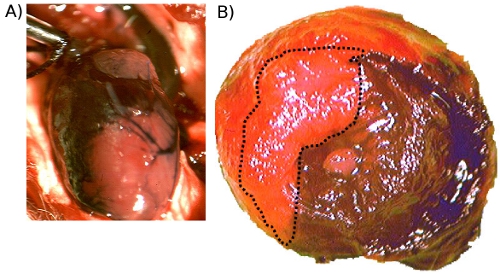
Figure 1. A. Evan’s Blue injected into the aorta prior to excision. This image shows the perfused regions of the heart (stained) and the occluded area (unstained). B. Evan’s Blue and TTC staining following acute ischemia/reperfusion injury. This is a representative image (20x) showing the blue dye distribution which stained the unoccluded regions as well as TTC staining of metabolically viable tissue (red). Necrotic areas do not stain and so they remain pale (outlined).
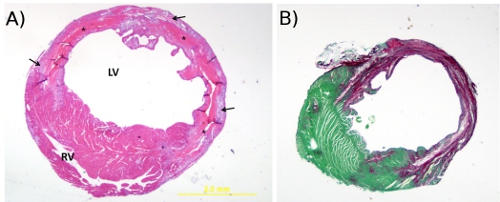
Figure 2. A. Hematoxylin and eosin stain. This is a representative image (20x) of H&E staining of a mouse heart cut transversely through the infarct region at 4 days post-MI (20x). The * denotes tissue necrosis, arrows point to granulation tissue, RV = right ventricle and LV = left ventricle. B. Picrosirius red and fast green stain. This is a representative image (20x) of picrosirius red/fast green staining of a cross-section of mouse heart at 4 weeks post-MI. The cytoplasm stains green and collagen fibers are red.
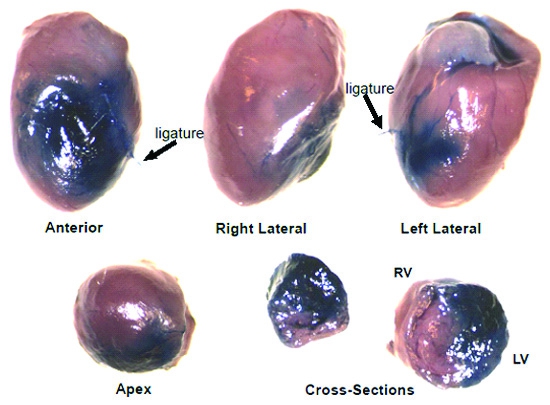
Figure 3. Evan’s Blue dye stain distribution following 6ul intramyocardial injection. This is a representative image showing the global and transmural distribution of Evan’s Blue dye throughout the heart following 6ul intramyocardial injection at the border zone immediately following coronary artery ligation (12x).
Discussion
Coronary heart disease continues to be an epidemiologically and fiscally significant public health problem. Considerable basic research is still needed to understand the mechanisms by which injury and remodeling proceed and how potential therapeutics may modulate these processes if they are to be developed for clinical use. Rodents are most commonly used and the wide range of genetically modified mice available makes this species a more attractive model.
Although there are differences between mice and other species, there are many advantages to a murine model. Use of a simple dissecting scope or magnifying glass and well lit conditions enable the vasculature to readily be seen (for detailed gross anatomy of vasculature, see Salto-Tellez et al., 200413). To reduce the risk of post-operative mortality, it is very important to avoid severing large vessels since the total blood volume of a 25g mouse is less than 2ml14. In the event that excessive bleeding occurs, gentle application of pressure or pinpoint cauterization can be used to stop the bleeding.
This procedure can also be modified in a variety of ways. For example, mice can be anesthetized using isoflurane, ketamine/xylazine, or sodium pentobarbital and appropriate selection is determined by the duration of the protocol15-18. The toe-pinch reflex is the most commonly used index of the depth of anesthesia. Further, to improve the probability for long term survival, some investigators use antiarrhythmic drugs such as lidocaine to reduce the incidence of lethal arrhythmias19,20 however, it must be taken into account that this has recently been shown to have antiapoptotic properties in an acute model21. Also, to reduce post-operative pain, analgesics such as buprenorphine can be administered for the first 48 hours after surgery3,16,17,22,23. To maintain body temperature during surgery (especially for longer protocols), a rectal probe in series with a heating pad is often used in place of the isothermal pad. For ischemia/reperfusion and/or ischemic pre- or postconditioning: the duration of the occlusion(s) and reperfusion(s) can be altered; for permanent occlusion, the size of the infarct may be modified by adjusting the location of the ligature; and for intramyocardial injections (eg. cells, proteins), there can be 1-3 injection locations and the volume per injection can be up to 15 μl24. If cells are being injected, the gauge of the needle used (usually 26-30)5,25,26 should be chosen based on the size of the cells so the internal diameter of the needle is large enough to avoid sheering. To avoid confounds due to inflammatory processes triggered by the surgery, some investigators have reported using a snare that is manipulated ex vivo to occlude and reperfuse the hearts in a closed chest mouse at any point after the surgery27-29. More recently, Gao et al.30 have shown that temporary and permanent occlusion can be performed without the need for ventilation and a few laboratories have begun to use ultrasound to perform closed-chest intramyocardial injections25,31.
Since the first study demonstrating feasibility of ligating the coronary artery in mice was published by Johns and Olson in 195432, many others have adopted this model and modified it to study various aspects of myocardial injury and remodeling3,33-45. The nature of mice in terms of size, reproductive capacity, and comparatively less expense for purchase and maintenance make this species an appealing tool for a broad range of physiologic and pathophysiologic studies. As the miniaturization of technology for imaging in vivo advances46-49, as well as means to perform and analyze large scale genomics and proteomics, drug screening, efficacy of cell-based and/or protein therapies as well as biomaterials50-64, combined with the increasingly wide range of genetic manipulations afforded by ubiquitous or tissue specific transgenic or mutant/knockout mice, the murine model of myocardial infarction will undoubtedly continue to be an invaluable tool in evaluating acute cardiac injury and long term remodeling. Therefore, there is unquestionable value in being able to perform these experiments reliably and reproducibly.
Disclosures
The authors have nothing to disclose.
Acknowledgements
I would like to acknowledge the Department of Research and Graduate Studies for providing funds to support my research and the Department of Comparative Medicine for their vigilance and assistance. I would also like to recognize the Department of Physiology for their support and guidance as well as the students and technicians in my lab for their help. Lastly, I would like to thank my post-doctoral mentor, Dr. Charles E. Murry, for the training opportunity during which time I learned the mouse microsurgery.
Materials
| Material Name | Type | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Long Vanna Scissors | George Tiemann | 160-159 | ||
| Micro Dissecting Scissors | George Tiemann | 160-161 | ||
| Forceps – straight, 1×2 teeth | George Tiemann | 105-205 | ||
| Scalpel handle #3 | George Tiemann | 105-64 | #10 sterile blade | |
| Forceps – half curved serrated | George Tiemann | 160-19 | ||
| Tissue Scissors | George Tiemann | 105-410 | ||
| Castroviejo Needle Holder | Miltex | 18-1828 | ||
| Cook Eye Speculum | Miltex | 18-63 | ||
| Surgipro II 8-0 | Suture Express | VP-900-X | ||
| Prolene 6-0 | Suture Express | 8776 | ||
| Germinator 500 Bead Sterilizer | Cellpoint Scientific | 65369-1 | ||
| Deltaphase isothermal pad | Braintree Scientific | 39DP | ||
| Hamilton syringe – 25μl | Hamilton | 80430 | ||
| 30 gauge beveled needle | Hamilton | 7803-07 | ||
| Ventilator | Kent Scientific | TOPO |
References
- Reinhard, C. Inbred strain variation in lung function. Mamm Genome. 13, 429-437 (2002).
- Schulz, H. Respiratory mechanics in mice: strain and sex specific differences. Acta Physiol Scand. 174, 367-375 (2002).
- Tarnavski, O. Mouse cardiac surgery: comprehensive techniques for the generation of mouse models of human diseases and their application for genomic studies. Physiol Genomics. 16, 349-360 (2004).
- Klocke, R., Tian, W., Kuhlmann, M. T., Nikol, S. Surgical animal models of heart failure related to coronary heart disease. Cardiovasc Res. 74, 29-38 (2007).
- Murry, C. E. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 428, 664-668 (2004).
- Nussbaum, J. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. Faseb J. 21, 1345-1357 (2007).
- Reinecke, H., Minami, E., Virag, J. I., Murry, C. E. Gene transfer of connexin43 into skeletal muscle. Hum Gene Ther. 15, 627-636 (2004).
- Virag, J. A. Attenuation of Myocardial Injury in Mice with Functional Deletion of the Circadian Rhythm Gene mPer2. Am J Physiol Heart Circ Physiol. , (2008).
- Virag, J. A. Fibroblast growth factor-2 regulates myocardial infarct repair: effects on cell proliferation, scar contraction, and ventricular function. Am J Pathol. 171, 1431-1440 (2007).
- Virag, J. I., Murry, C. E. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 163, 2433-2440 (2003).
- Cozzi, E. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol. 291, 894-903 (2006).
- Virag, J. A. Attenuation of myocardial injury in mice with functional deletion of the circadian rhythm gene mPer2. Am J Physiol Heart Circ Physiol. 298, 1088-1095 (2010).
- Salto-Tellez, M. Myocardial infarction in the C57BL/6J mouse: a quantifiable and highly reproducible experimental model. Cardiovasc Pathol. 13, 91-97 (2004).
- Diehl, K. H. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 21, 15-23 (2001).
- Lichtenberger, M., Ko, J. Anesthesia and analgesia for small mammals and birds. Vet Clin North Am Exot Anim Pract. 10, 293-315 (2007).
- Davis, J. A. Mouse and rat anesthesia and analgesia. Curr Protoc Neurosci. Appendix 4, Appendix 4B-Appendix 4B (2008).
- Flecknell, P. A. Anaesthesia of animals for biomedical research. Br J Anaesth. 71, 885-894 (1993).
- Kolk, M. V. LAD-ligation: a murine model of myocardial infarction. J Vis Exp. , (2009).
- Kinoshita, H. T-type Ca2+ channel blockade prevents sudden death in mice with heart failure. Circulation. 120, 743-752 (2009).
- Mulder, P. Increased survival after long-term treatment with mibefradil, a selective T-channel calcium antagonist, in heart failure. J Am Coll Cardiol. 29, 416-421 (1997).
- Kaczmarek, D. J. Lidocaine protects from myocardial damage due to ischemia and reperfusion in mice by its antiapoptotic effects. Anesthesiology. 110, 1041-1049 (2009).
- Blaha, M. D., Leon, L. R. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci. 47, 8-19 (2008).
- Flecknell, P. A. Analgesia of small mammals. Vet Clin North Am Exot Anim Pract. 4, 47-56 (2001).
- Dries, J. L., Kent, S. D., Virag, J. A. Intramyocardial administration of chimeric ephrinA1-Fc promotes tissue salvage following myocardial infarction in mice. J Physiol. 589, 1725-1740 (2011).
- Springer, M. L. Closed-chest cell injections into mouse myocardium guided by high-resolution echocardiography. Am J Physiol Heart Circ Physiol. 289, 1307-1314 (2005).
- Wang, C. C. Direct intramyocardial injection of mesenchymal stem cell sheet fragments improves cardiac functions after infarction. Cardiovasc Res. 77, 515-524 (2008).
- Nossuli, T. O. A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol. 278, 1049-1055 (2000).
- Kim, S. C. Toll-like receptor 4 deficiency: smaller infarcts, but no gain in function. BMC Physiol. 7, 1472-6793 (2007).
- Fazel, S. S. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. Faseb J. 22, 930-940 (2008).
- Gao, E. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 107, 1445-1453 (2010).
- Fujii, H. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc Imaging. 2, 869-879 (2009).
- Johns, T. N., Olson, B. J. Experimental myocardial infarction. I. A method of coronary occlusion in small animals. Ann Surg. 140, 675-682 (1954).
- Frangogiannis, N. G. The immune system and cardiac repair. Pharmacol Res. 58, 88-111 (2008).
- Dobaczewski, M., Frangogiannis, N. G. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed). 1, 391-405 (2009).
- Willis, M. S., Townley-Tilson, W. H., Kang, E. Y., Homeister, J. W., Patterson, C. Sent to destroy: the ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 106, 463-478 (2010).
- Nithipatikom, K., Gross, G. J. Review article: epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther. 15, 112-119 (2010).
- Palaniyandi, S. S., Sun, L., Ferreira, J. C., Mochly-Rosen, D. Protein kinase C in heart failure: a therapeutic target. Cardiovasc Res. 82, 229-239 (2009).
- Michael, L. H. Myocardial ischemia and reperfusion: a murine model. Am J Physiol. 269, 2147-2154 (1995).
- Patten, R. D. Ventricular remodeling in a mouse model of myocardial infarction. Am J Physiol. 274, 1812-1820 (1998).
- Paigen, K. A miracle enough: the power of mice. Nat Med. 1, 215-220 (1995).
- Kogan, M. E., Belov, L. N., Leont’eva, T. A., Zolotareva, A. G. Modeling of myocardial pathology in mice with the surgical methods. Kardiologiia. 17, 125-128 (1977).
- Tarnavski, O. Mouse surgical models in cardiovascular research. Methods Mol Biol. 573, 115-137 (2009).
- Wong, R., Aponte, A. M., Steenbergen, C., Murphy, E. Cardioprotection leads to novel changes in the mitochondrial proteome. Am J Physiol Heart Circ Physiol. 298, 75-91 (2010).
- Dobaczewski, M., Gonzalez-Quesada, C., Frangogiannis, N. G. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 48, 504-511 (2010).
- Zhao, Z. Q. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 285, 579-588 (2003).
- Thibault, H. Acute myocardial infarction in mice: assessment of transmurality by strain rate imaging. Am J Physiol Heart Circ Physiol. 293, 496-502 (2007).
- Scherrer-Crosbie, M. Three-dimensional echocardiographic assessment of left ventricular wall motion abnormalities in mouse myocardial infarction. J Am Soc Echocardiogr. 12, 834-840 (1999).
- Stypmann, J. Echocardiographic assessment of global left ventricular function in mice. Lab Anim. 43, 127-137 (2009).
- Pistner, A., Belmonte, S., Coulthard, T., Blaxall, B. Murine echocardiography and ultrasound imaging. J Vis Exp. , (2010).
- Zimmermann, W. H. Heart muscle engineering: an update on cardiac muscle replacement therapy. Cardiovasc Res. 71, 419-429 (2006).
- Mangi, A. A. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 9, 1195-1201 (2003).
- Chavakis, E., Koyanagi, M., Dimmeler, S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 121, 325-335 (2010).
- Mirotsou, M., Jayawardena, T. M., Schmeckpeper, J., Gnecchi, M., Dzau, V. J. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. , (2010).
- Fromstein, J. D. Seeding bioreactor-produced embryonic stem cell-derived cardiomyocytes on different porous, degradable, polyurethane scaffolds reveals the effect of scaffold architecture on cell morphology. Tissue Eng Part A. 14, 369-378 (1089).
- Lee, R. J. Stem cells for myocardial repair and regeneration: where are we today. Methods Mol Biol. 660, 1-6 (2010).
- Segers, V. F., Lee, R. T. Protein Therapeutics for Cardiac Regeneration after Myocardial Infarction. J Cardiovasc Transl Res. , (2010).
- Webber, M. J. Capturing the stem cell paracrine effect using heparin-presenting nanofibres to treat cardiovascular diseases. J Tissue Eng Regen Med. 10, (2010).
- Kofidis, T. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 112, I173-1177 (2005).
- Vunjak-Novakovic, G. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev. 16, 169-187 (2010).
- Guyette, J. P., Cohen, I. S., Gaudette, G. R. Strategies for regeneration of heart muscle. Crit Rev Eukaryot Gene Expr. 20, 35-50 (2010).
- Menasche, P. Cardiac cell therapy: Lessons from clinical trials. J Mol Cell Cardiol. , (2010).
- Ott, H. C., McCue, J., Taylor, D. A. Cell-based cardiovascular repair–the hurdles and the opportunities. Basic Res Cardiol. 100, 504-517 (2005).
- Terrovitis, J. V., Smith, R. R., Marban, E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 106, 479-494 (2010).
- Nelson, T. J. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 120, 408-416 (2009).

