Transplantation of Cells Directly into the Kidney of Adult Zebrafish
Summary
Cell transplantation is an essential technique for studying tissue regeneration and for developing cell-based therapies of disease. We demonstrate here a microsurgical technique that permits the transplantation of genetically labeled cells directly into the kidney of adult zebrafish fish.
Abstract
Regenerative medicine based on the transplantation of stem or progenitor cells into damaged tissues has the potential to treat a wide range of chronic diseases1. However, most organs are not easily accessible, necessitating the need to develop surgical methods to gain access to these structures. In this video article, we describe a method for transplanting cells directly into the kidney of adult zebrafish, a popular model to study regeneration and disease2. Recipient fish are pre-conditioned by irradiation to suppress the immune rejection of the injected cells3. We demonstrate how the head kidney can be exposed by a lateral incision in the flank of the fish, followed by the injection of cells directly in to the organ. Using fluorescently labeled whole kidney marrow cells comprising a mixed population of renal and hematopoietic precursors, we show that nephron progenitors can engraft and differentiate into new renal tissue – the gold standard of any cell-based regenerative therapy. This technique can be adapted to deliver purified stem or progenitor cells and/or small molecules to the kidney as well as other internal organs and further enhances the zebrafish as a versatile model to study regenerative medicine.
Protocol
1. Conditioning of non-transgenic recipient fish.
- Select the biggest male fish in the system for transplantation.
- Place the fish in 0.02% Tricaine (pH 7) for approximately 3 minutes or until it stops swimming, but not so long that the heart stops beating.
- Put the anesthetized fish on a damp paper towel on a bench top.
- Use an insulin syringe needle to inject 40 ug of gentamicin in 20 uL of water into the intraperitoneal cavity of the fish. For renal regeneration studies, this step is important for inducing a favorable environment for enhanced engraftment.
- Put the fish back into system water for recovery.
- The fish may be docile and very sensitive to external stimulus such as vibration. After 3 hours of recovery, treat the fish with 25 grey of gamma irradiation.
- Put the fish back into the system for 3 days without food prior to transplantation.
2. Preparation of progenitor donor cells genetically labeled with a fluorescent marker (EGFP or mCherry).
- Just prior to the surgical procedure, prepare the donor cells to be transplanted and keep them on ice.
- For renal studies, we use whole kidney marrow cells from the Tg (cdh17:EGFP) transgenic line, which contain renal and hematopoietic progenitor cells. These progenitor cells are genetically labeled and will only express EGFP if they engraft and differentiate into renal epithelial cells. Other types of labeled progenitor cells can also be used, such as Tg(scl:EGFP) for hematopoietic stem cell or Tg(fli1a:EGFP) for angioblast progenitors transplants.
- Euthanize 3 adult transgenic fish by placing them in 0.2% Tricaine for 5 minutes.
- Use a sterilized razor blade to remove the head and tail, and dissecting scissors to make a midline incision along the ventral side.
- Under a stereoscope, remove all of the internal organs in the body cavity including the swim bladder with tweezers. Be careful not to remove the kidney, which is a flat and pigmented organ attached to the dorsal wall of the cavity.
- Next, dissect out the kidney from the dorsal wall and place it into a 1.5 mL centrifuge tube containing 300 uL of cold phosphate buffer saline supplemented with 2% fetal calf serum (1X PBS/2% FCS).
- Use a pestle to shear the kidney until it becomes homogenized.
- Place a 40 uM cell strainer inside a 50 mL Falcon tube and transfer the cell solution on top of the cell strainer, allowing the loose cells to filter through into the Falcon tube.
- Use the pestle to gently smear the big pieces of tissue that are left on the strainer to further break up the cells.
- Leave the cell solution in the Falcon tube and wash the strainer with 1.2 mL of 1X PBS/2% FCS to collect residual cells from the strainer.
- Transfer the cell solution into a new 1.5 mL tube and centrifuge at 4°C at 300 rcf for 5 minutes.
- Wash the cell pellet with 1.5 mL of 1X PBS/2% FCS and pass it through a new strainer to filter out clumps of sticky cells and centrifuge again.
- Resuspend the cell pellet in 200 uL of of 1X PBS/2% FCS and transfer to a PCR tube.
- Centrifuge the PCR tube (4°C, 300 rcf, 1 min) and remove most of supernatant.
- Resuspend the cells in the residual supernatant to get a 2-5 uL final volume and store it on ice.
- Final cellular yield should be approximately 2 million cells per kidney.
- Count the number of cells with a hemocytometer and adjust the concentration to 5 x 105 cells per uL.
3. Load the cell solution into a Hamilton syringe.
- Rinse the Hamilton syringe with sterile water 3 times.
- Rinse the syringe with 75% ethanol 3 times.
- Rinse the syringe again with 1X PBS/2% FCS 3 times.
- Then load the syringe with 1 uL of cell solution contain approximately 5 x 105 cells just prior to injection.
4. Transplantation of progenitor cells directly into the kidney.
- Anesthetize the conditioned recipient fish in 0.02% Tricaine for about 3 minutes, or until the fish has stopped moving, but not too long that the heart stops beating.
- Under a stereomicroscope, lay the fish on a paper towel with the head towards the left, ventral side up, in order to dry off the transplanted side.
- Then roll the fish over to expose the dried side.
- Using sterilized tweezers, remove the scales in the region immediately posterior to the gills.
- Make a 1 cm lateral incision in this area at the midline with a scalpel while stabilizing the tissue with tweezers.
- Using the tweezers, expose the tissue while continuing to make the incision deep enough to expose the head kidney.
- If there is a lot of bleeding that prevents visualization of the kidney, euthenize the fish and start over with another recipient fish.
- While prying the tissue open to expose the head kidney, inject 1 uL of the cell solution directly into the head kidney.
- Then, at the same time as the syringe needle is being retrieved, release the tweezers so that the tissue falls back into place to prevent leaking of the injected cells.
- Hold a suture needle with a needle driver and pierce it through the muscle on each side of the incision.
- Tie 2 knots and cut off the excess suture. Only a single suture is needed for a 1 cm incision.
- Place the fish in fish water containing 10 parts per million of Tricaine for 5 hours to provide fish with a calming analgesic effect.
- Place the fish back into the system for recovery with normal feedings.
- After 7-18 days of recovery, we dissect the fish and analyze engraftment of renal progenitor cells by live EGFP fluorescence.
5. Representative Results:
The overall strategy of the transplantation is illustrated in Figure 1. For our renal studies, the kidney is dissected and analyzed by EGFP fluorescence. Engraftment and differentiation of renal progenitor cells is detected as early as seven days post transplant. This is evident by the presence of multiple clusters of EGFP+ renal epithelial cells (Figure 2). By 18 days post transplant, we detect an average of 24 EGFP+ nephrons, indicating that donor cells have formed new kidney tissue (Figure 3). To demonstrate long-term engraftment, we dissected a recipient fish 59 days post transplant (Figure 4). This fish had many nephrons at the site of injection (red arrowhead) in addition to multiple nephrons at distant sites (white arrowheads), suggesting that renal progenitor cells are migratory. This technique is faster and more efficient compared to when cells are injected into the circulation via the heart, which takes approximately three months to detect engraftment.
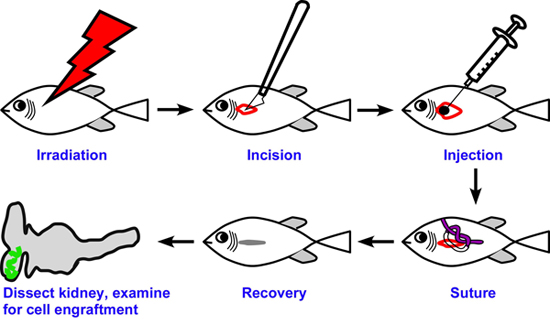
Figure 1. Overall scheme of transplantation. Non-transgenic recipient fish are irradiated to suppress immune rejection of transplanted cells. Three days later, an incision is made on the flank of the fish and donor progenitor cells are injected into the exposed head kidney. The incision is closed up with one suture and fish are placed back into the system for recovery. Kidneys of recipient fish are dissected at later time points and analyzed for engraftment and progenitor activity.
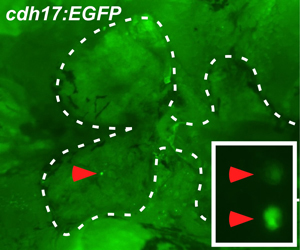
Figure 2. Engraftment of progenitor cells after seven days. Engraftment of renal progenitor cells is detected on the injected side of the head kidney at seven days post transplant, as determined by the presence of clusters of EGFP+ renal epithelial cells (inset – magnified view).
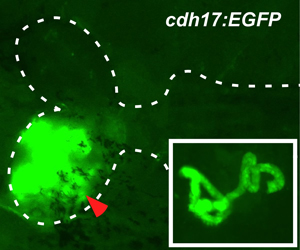
Figure 3. Engraftment of progenitor cells after 18 days. After 18 days post transplant, we detect an average of 24 EGFP+ donor-derived nephrons (insert – magnified view of individual EGFP+ nephrons).
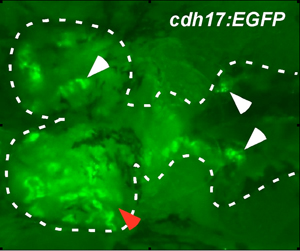
Figure 4. Engraftment and migration of progenitor cells after 59 days. We continued to detect engraftment of progenitor cells long after transplantation, even after 59 days (red arrow head). At this later time point, donor-derived nephrons are detected at sites away from the site of injection (white arrow heads), consistent with progenitor cell migration.
Discussion
Cell transplantation techniques are crucial for regenerative studies and are the gold standard assay for measuring stem cell activity. Here, we report a method for transplanting cells directly into the kidney of adult zebrafish to determine stem and progenitor cell activity. This procedure involves making an incision through the muscle to expose the head kidney, followed by injection of cells directly into the kidney. The incision is closed with a single suture and the operated fish have a 90-100% survival rate. In our experiments, engrafted renal progenitor cells are detected in 80-100% of the transplanted fish by seven days post transplantation. One limitation of this technique is that the incision is near the dorsal aorta, which can be easily ruptured by the scalpel. However, once mastered, the rate of bleeding can be reduced to less than one percent. Our method could be adapted to deliver other cell types (blood, stromal, or vascular) as well as small molecules to the kidney. The development of this technique will help advance regenerative studies and permit stem and progenitor cell potential to be assessed in vivo.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank E.C. Liao for help with suturing, and R. Ethier and L. Gyr for zebrafish care. A.J.D. was supported by the Harvard Stem Cell Institute, the American Society of Nephrology, and NIH/NIDDK (P50DK074030). C.Q.D. was supported by the Massachusetts General Hospital-Fund for Medical Discovery.
Materials
| Name of the reagent | Company | Catalogue number |
|---|---|---|
| Insulin syringe needle (28 gauge) | Becton Dickinson | 329461 |
| Cell strainer (40 uM) | Becton Dickinson | 352340 |
| Tricaine | Sigma | A5040 |
| Hamilton syringe (26 gauge) | Sigma | 20734 |
| Ethilon suture (size 9-0) | Ethicon | 2819G |
References
- Hopkins, C., Li, J., Rae, F., Little, M. H. Stem cell options for kidney disease. The Journal of Pathology. 217, 265-281 (2009).
- Brittijn, S. Zebrafish development and regeneration: new tools for biomedical research. Int J Dev Biol. 53, 835-850 (2009).
- Traver, D. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood. 104, 1298-1305 (2004).

