HLA-Ig Based Artificial Antigen Presenting Cells for Efficient ex vivo Expansion of Human CTL
Summary
A new DC independent method for induction and expansion of antigen-specific T cells is described. HLA A2-Ig based artificial Antigen Presenting Cells (aAPC) are loaded with HLA-A2 restricted peptides to efficiently expand CTL of diverse antigen specificity. This technology holds great potential for CTL-based adoptive immunotherapy.
Abstract
CTL with optimal effector function play critical roles in mediating protection against various intracellular infections and cancer. However, individuals may exhibit suppressive immune microenvironment and, in contrast to activating CTL, their autologous antigen presenting cells may tend to tolerize or anergize antigen specific CTL. As a result, although still in the experimental phase, CTL-based adoptive immunotherapy has evolved to become a promising treatment for various diseases such as cancer and virus infections. In initial experiments ex vivo expanded CMV (cytomegalovirus) specific CTL have been used for treatment of CMV infection in immunocompromised allogeneic bone marrow transplant patients. While it is common to have life-threatening CMV viremia in these patients, none of the patients receiving expanded CTL develop CMV related illness, implying the anti-CMV immunity is established by the adoptively transferred CTL1. Promising results have also been observed for melanoma and may be extended to other types of cancer2.
While there are many ways to ex vivo stimulate and expand human CTL, current approaches are restricted by the cost and technical limitations. For example, the current gold standard is based on the use of autologous DC. This requires each patient to donate a significant number of leukocytes and is also very expensive and laborious. Moreover, detailed in vitro characterization of DC expanded CTL has revealed that these have only suboptimal effector function 3.
Here we present a highly efficient aAPC based system for ex vivo expansion of human CMV specific CTL for adoptive immunotherapy (Figure 1). The aAPC were made by coupling cell sized magnetic beads with human HLA-A2-Ig dimer and anti-CD28mAb4. Once aAPC are made, they can be loaded with various peptides of interest, and remain functional for months. In this report, aAPC were loaded with a dominant peptide from CMV, pp65 (NLVPMVATV). After culturing purified human CD8+ CTL from a healthy donor with aAPC for one week, CMV specific CTL can be increased dramatically in specificity up to 98% (Figure 2) and amplified more than 10,000 fold. If more CMV-specific CTL are required, further expansion can be easily achieved by repetitive stimulation with aAPC. Phenotypic and functional characterization shows these expanded cells have an effector-memory phenotype and make significant amounts of both TNFα and IFNγ (Figure 3).
Protocol
1. Making, HLA-A2-Ig based aAPC
- Prepare sterile borate buffer
- Dissolve boric acid in water to make 0.1M. Adjust the pH to 7.0.
- Filter through a 0.22μm sterile filter and store at 4°C.
- Prepare sterile Bead Wash buffer
- Take 956ml PBS w/o magnesium or calcium.
- Add 30ml human AB serum.
- Add EDTA 2mM final concentration.
- Add 0.1gsodium azide.
- Filter through a 0.22 μm sterile filter and store at 4°C.
- Take 1ml of Invitrogen M-450 Epoxy beads from stock, approximately 400 million beads (beads can be counted by hemocytometer), and put into a sterile, screw top glass vial.
- Put the vial against a Dynal magnet MPC-1, while the beads adhere to the side of the vial, remove the supernatant by aspiration. Wash beads once with 1ml of borate buffer.
- Resuspend beads in a mixture of 1ml borate buffer and 20 μg of HLA-A2-Ig dimer and 20 μg of anti-human CD28 mAb (Clone 9.3).
- Protein to bead conjugation: put the glass vial on rotator and rotate at 4°C for 24 hours.
- Place the tube in MPC-1 magnet and remove all borate buffer.
- Wash the beads twice with 1ml Bead Wash buffer.
- Incubate the beads in 1ml Bead Wash buffer, rotate at 4°C for 24 hours. Because Bead Wash buffer contains human AB serum, it will block all the residual protein binding site on the beads.
- Remove the supernatant from glass vial, replace with 1ml fresh Bead Wash buffer.
2. Quality control of aAPC and peptide loading, storage
- Add ˜5×105 beads to 100μl FACS washing buffer in FACS tubes, and stain with 1μl of anti-mouse IgG1-PE (recognize the Fc part of HLA-A2-Ig) and 1μl of anti-mouse IgG2a-FITC (recognize the Fc part of anti-CD28 mAb). After staining for 20 minutes in FACS washing buffer, wash again and read the staining result immediately by flow cytometer (Figure 4).
- Peptide loading onto the beads: wash the beads twice in glass vial with 1ml sterile PBS. Resuspend with 1ml sterile PBS then add 10μl of CMV peptide (1mg/ml)
- Count the beads by hemocytometer, and label the vial with date and concentration.
- aAPC are ready for use after at least 3 days peptide incubation at 4°C, to allow sufficient time of peptide binding onto the HLA-A2-Ig dimer. The beads can be stored at 4°C and remain functional for at least 6 month.
3. Human CTL isolation
- Collect ˜100ml of fresh peripheral blood from healthy HLA-A2 positive donor into 10 BD Sodium Heparin Vacutainer tubes. Use a 21-gauge needle or larger to prevent hemolysis.
- Centrifuge at 300xg for 10 minutes at room temperature
- Carefully remove the top plasma layer by aspiration. The plasma can be used as supplement for the culture medium.
- Replace the collected plasma with sterile PBS and transfer the blood into a sterile T75 culture flask or 50ml conical tubes. Mix the blood with PBS well by pipetting up and down.
- Once all the blood is collected, prepare four additional 50ml conical tubes and add 15ml of Ficoll-Paque Plus.
- Slowly overlay 30-35ml of blood cells on top of the Ficoll of each tube. Keep the interface distinct between the Ficoll and blood cells.
- Centrifuge at 500xg for 20 minutes at room temperature. Turn the brake “off” and the acceleration as low as possible to maintain a clear interface between the layers.
- Using a seriological pipette, carefully aspirate the PBMC layer and collect the PBMC into a fresh 50ml conical tube. When all PBMC are harvested add 30ml of PBS and spin at 400xg for 10 min. Discard the supernatant and wash one more time with 30ml PBS to remove all residual Ficoll.
- Proceed to CD8+ T cell isolation by using Miltenyi human CD8+ T cell isolation kit according to manufacturer’s protocol. This kit highly enriches for CD8+ cells (usually >95%) by depleting CD8– cells.
- Count the CD8+ T cells. The expected purity should be greater than 95%. To confirm this use 2×105 cells and perform a CD4/3/8 FACS analysis. The remaining CTL can be either used right away for antigen-specific aAPC stimulation or they can be frozen for future use.
4. In vitro aAPC based culture system
- Prepare culture medium
- For TF (T cell growth factor, made in the lab4) 2X culture medium: complete RPMI medium plus 5% donor autologous plasma and 8% T cell growth factor.
- Donor autologous plasma can be substituted by heat-inactivated human AB serum.
- Resuspend 1 million CTL in 8ml of TF 2X culture medium plus 8ml of complete RPMI medium, add 1×106 aAPC, mix well.
- Use a multi-channel pipetter to plate cells onto 96 well U bottom tissue culture plates. (160 μl per well)
- Culture cells in 37°C, 5% CO2 incubater for 7 days. Feed the cells on day 4 with 80 μl/well TF 2X medium.
- Cells are ready to be harvested on day 7. After harvest, place the cells against the magnet and remove the old aAPC.
- Antigen specificity can be determined by tetramer staining according to manufacturer’s protocol. Phenotype staining and intracellular cytokine staining are performed according to our previous study3.
- Harvested cells can be replated with aAPC again under the same conditions. Cell number and antigen specificity is expected to increase after repeated stimulation.
5. Representative Results:
An example of aAPC after HLA A2-Ig and anti-CD28 conjugation is shown in Figure 4. Successful protein conjugation is evident by a clear shift of corresponding antibody staining. While the frequency of CMV specific CTL in the peripheral blood is typically 0.5-1%, after a single week of aAPC-mediated stimulation, the specificity can reach 55- 93% (Figure 2 and 3). The expansion of antigen specific CTL can be very variable between different donors, but the results are reproducible within the same donor. By extrapolation, the expansion of CMV specific cells can be thousands of fold compared with precursor levels directly ex vivo (data not shown). Intracellular cytokine staining (Figure 3) shows that these expanded CTL are polyfunctional, rather than exhausted, after prolonged cell culture and significant proliferation.
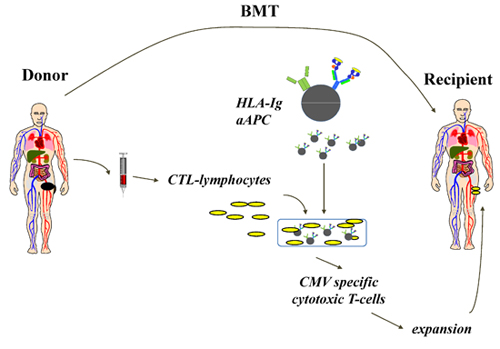
Figure 1. Representative flow chart of aAPC based ex vivo expansion of human CTL for adoptive immunotherapy in allogeneic HSCT
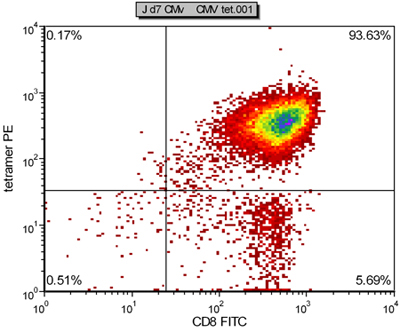
Figure 2. Representative tetramer staining result of CMV specific CTL generated by aAPC after one week of culture
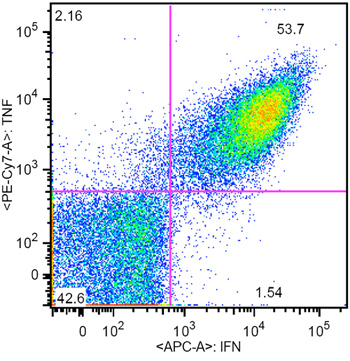
Figure 3. Representative intracellular cytokine staining result of CMV specific CTL generated by aAPC (CMV specificity was 61%)
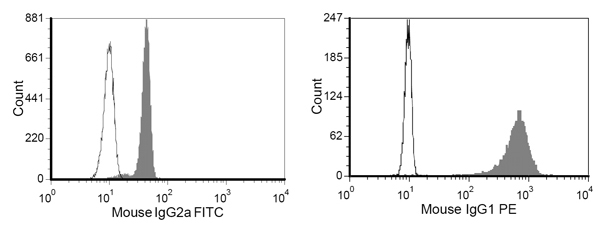
Figure 4. Representative staining result of M-450 Epoxy beads after protein conjugation stained with anti-mouse IgG1-PE and anti-mouse IgG2-FITC
Discussion
The aAPC system we describe here is an efficient system for ex vivo expansion of human CTL against a variety of antigens. Special care should be taken with regards to the quality of protein conjugation and the even distribution of aAPC and CTL in the 96-well plate culture. Using this approach we have been able to expand CTL for more than 8 weeks, during which we expanded antigen-specific CTL up to a million fold4. There have been various artificial APC systems utilizing cell lines or other acellular platforms5; however, according to the published data every system has its unique profile with regards to expansion and specificity supporting different applications. Importantly, since the quality of CTL is as important as quantity, the polyfunctionality of the CMV-specific CTL generated by our system are expected to confer superior anti-viral efficiency.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Aaron Selya for helpful discussion. This work was supported by NIH grant AI29575, CA108835, AI077097 to JS, a pilot grant from the Johns Hopkins Malaria Research Institute and the Department of Defense grant PC 040972 to M.O.
Materials
| Reagent | Company | Catalogue number |
|---|---|---|
| Vacutainer tube (contains heparin) | Becton Dickinson | 367874 |
| Human CD8+ T cell isolation kit | Miltenyi | 130-094-156 |
| Dynabeads M-450 Epoxy | Invitrogen | 140.11 |
| Dynal MPC-1 Magnet | Invitrogen | 120-01D |
| Ficoll-Paque Plus | GE healthcare | 17-1440-03 |
| RPMI medium 1640 | Gibco | 11875 |
| HLA-A2-Ig dimer X | Becton Dickinson | 551263 |
| iTAgMHC tetramer (HLA-A2-CMV)-PE | Beckman Coulter | T20099 |
| Falcon clear 96-well Microtest plate | Becton Dickinson | 353077 |
| Rat anti-mouse IgG2a-FITC | Becton Dickinson | 553390 |
| Goat anti-mouse IgG1-PE | Invitrogen | P21129 |
| Human serum type AB | Atlanta biologicals | S40110 |
| Mouse anti-human CD8a-FITC | Sigma-Aldrich | F0772 |
| Mouse anti-human CD8a-APC | Becton Dickinson | 340684 |
| Mouse anti-human IFNγ-FITC | Becton Dickinson | 340449 |
| Mouse anti-human TNFα-PE | Becton Dickinson | 340512 |
References
- Walter, E. A. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 333, 1038-1038 (1995).
- Rosenberg, S. A. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 8, 3669-3669 (2008).
- Oelke, M. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 9, 619-619 (2003).
- Oelke, M. Artificial antigen-presenting cells: artificial solution for real diseases. Trends Mol Med. 11, 412-412 (2005).

