An Optimized Protocol for Rearing Fopius arisanus, a Parasitoid of Tephritid Fruit Flies
Summary
Fopius arisanus is an egg-larval parasitoid of Tephritid fruit flies that is successfully used in biological control of these important tropical pests. We describe here an optimized protocol for rearing F. arisanus on a small scale using readily available materials.
Abstract
Fopius arisanus (Sonan) is an important parasitoid of Tephritid fruit flies for at least two reasons. First, it is the one of only three opiine parasitoids known to infect the host during the egg stage1. Second, it has a wide range of potential fruit fly hosts. Perhaps due to its life history, F. arisanus has been a successfully used for biological control of fruit flies in multiple tropical regions2-4. One impediment to the wide use of F. arisanus for fruit fly control is that it is difficult to establish a stable laboratory colony5-9. Despite this difficulty, in the 1990s USDA researchers developed a reliable method to maintain laboratory populations of F. arisanus10-12. There is significant interest in F. arisanus biology13,14, especially regarding its ability to colonize a wide variety of Tephritid hosts14-17; interest is especially driven by the alarming spread of Bactrocera fruit fly pests to new continents in the last decade18. Further research on F. arisanus and additional deployments of this species as a biological control agent will benefit from optimizations and improvements of rearing methods. In this protocol and associated video article we describe an optimized method for rearing F. arisanus based on a previously described approach12. The method we describe here allows rearing of F. arisanus in a small scale without the use of fruit, using materials available in tropical regions around the world and with relatively low manual labor requirements.
Protocol
1. Prepare host fruit fly eggs for parasitization
- Prepare a substrate for fruit fly eggs to be parasitized by preparing agar (alternatively, Gelcarin GP812, FMC Biopolymer, Ewing NJ; a more cost effective option) filled dishes, 10 cm in length per side and 1.5 cm depth. Prepare agar at a concentration of 9 g per liter of water.
- Fill dishes to the rim with liquid agar (approximately 70ml), and allow them to cool and solidify (at least 45 minutes).
- Once the agar blocks are solid, apply a single layer of tissue paper (a single ply of Georgia Pacific ‘Preference’ batch tissue), covering the top of each block. The tissue should stick easily to the agar because of surface moisture.
- Apply a 0.5 ml volume fruit fly eggs suspended in water to the tissue covered surface of the agar blocks. In our insectary, this is equivalent to about 6000 eggs of Bactrocera dorsalis (12000 eggs per ml).
- Use a clean 2.5 cm wide brush dipped in water to spread the fruit fly eggs evenly over the tissue surface.
- Place the fruit fly eggs under the bottom screen of the parasitoid cages to allow oviposition. Tap the underside of the bottom screen to remove dead parasites which may obstruct the eggs. It is best if the bottom screen does not touch the eggs.
- Allow the parasites to oviposit overnight and into the next day, approximately 21 hours. This is sufficient time for a high percentage of the eggs to be parasitized. Longer exposure risks superparasitization.
2. Hatching and pupation of parasitized host fruit flies
- Prepare rearing container by placing 3 to 3.5 prepared agar blocks of parasitized eggs per 1.25 liters of fruit fly diet19 with eggs facing upward.
- Place the diet container with eggs on a riser over a 1.5 cm deep layer of fine grade vermiculite or washed sand in a larger pupation container. Cover the pupation container with a lid and tape the edges of the with masking tape. Vermiculite may be reused after sifting (see below).
- Ensure that there is sufficient space between the top of the diet container and the pupation container lid for fruit fly larvae to crawl out and ‘pop’20 into the vemiculite below.
- Move pupation containers to a room at 27°C and 80 % relative humidity (RH) for one week. Cover containers with a dark cloth for the first four days then remove the cloth for the remaining days.
- Remove larval diet after one week, once larvae have popped and entered the vermiculite for pupation. If there are larvae in the vermiculite, allow them to pupate overnight.
- Sieve the pupae from the vermiculite. Start with a coarse sieve (approximately 3 mm) then use a hand sieve to remove any remaining clumps of vermiculite.
3. Sorting and selection
- Pupa containing F. arisanus can be partially segregated from unparasitized fruit flies by size. Collect pupa between 1.65 and 2.26 mm in diameter, as these will be enriched for parasitized flies.12 Puparia which are smaller contain mostly undersized parasitoids, larger pupa contain mostly flies. Note also that for the parasitized pupae the percentage of females correlates positively with size12.
- A variety of methods may be employed to sort pupae by size. We will describe a mechanical method using a custom size sorter21.
- Place pupae in the funnel at the top of a vibration feeder which slowly feeds the size sorter.
- Feed the pupae into the size sorter, which consists of a pair of slightly divergent rolling bars at a 30° angle. The pupae drop individually by size into an array of slots that empty into ten separate containers through a funnel.
- Collect pupae from the cups in the size range of 1.65 to 2.26 mm and place them in an emergence cage for 7 more days until most flies have emerged from the unparasitized pupae.
- Next, use a fan to separate empty puparia from those still containing insects.
4. F. arisanus emergence and maintenance
- To maintain the colony of F. arisanus use a parasitoid holding cage with a removable glass front, approximately 25 cm in length per side. The cage has 1mm2 screening on the top and two of the sides. The back of the cage is covered in a rubber sheet with a 9 cm hole for access. The bottom of the cage has a cut out covered on the inside with 1-2mm2 screen large enough to allow placement of two agar blocks with fruit fly eggs for parasitoid oviposition.
- The holding cages should also have a tinted portion along the bottom quarter of the glass front to minimize light which can create parasitoid crowding along the front of the cage, resulting in elevated mortality.
- Place approximately 11 g of these selected pupae into plastic containers approximately 9 cm in diameter. This should produce about 600-700 parasites per cage. Size selection should result in about 60% females.
- Attach screened lids to the containers. The lids should be covered with 2 mm2 screening, which will allow adult F. arsianus to leave the containers upon emergence but, will contain any remaining fruit flies.
- Maintain the parasites at 24°C and about 45% RH with a 12:12 photoperiod and good ventilation. If high mortality is observed, moving the cages outdoors for a few hours a day or increasing ventilation may reduce it. Parasites should be ready to oviposit in one additional week and should be productive for two more weeks.
- Streak undiluted spun honey along the top of the cages at least three times per week or whenever the available honey is dry. Apply the spun honey in narrow streaks using a fingertip. Note that if the streaks are too heavy the honey will drip into the cage; if too light the honey will dry out quickly and require frequent reapplication.
- Place agar blocks (10 cm x 10 cm x 4 cm) on the tops of the cages to provide moisture to the parasites. These should be changed two times per week.
5. Representative Results:
We report here results from quality control procedures conducted on the USDA-ARS F. arisanus rearing operation in Hilo, Hawaii between January 5 and June 22, 2010. Rearing at the Hilo location on the scale described in this video article and protocol was initiated in August 2009, and initial problems and adjustments with the new location had been mostly resolved by January. Therefore, these data are representative of the results other researchers might obtain starting their colony if they follow the protocol as described and have experience rearing insects generally. During this period we were maintaining a small colony of F. arisanus: 4 parasitoid holding cages produced per week, equivalent to about 3600 F. arisanus.
An initial check of enriched parasitization rates was conducted by taking a 2 g sample of pupae in the size range of 1.65 – 2.26 mm (enriched) before an additional week of development in a holding cage (i.e. immediately after the larval diet was removed and the pupae sifted and size sorted. We refer to these as ‘early pupae’). During the first three months of 2010 the mean proportion parasites in the enriched sample was 0.46 (SD=0.18). During the following quarter this mean was 0.58 (SD=0.08), reflecting stabilization of the colony and rearing procedures at the new location. Note that the enriched pupae which were not successfully parasitized include those which did not produce emergents and those that produced flies.
After an additional week in the holding cage removal of empty puparia another two gram sample was taken (‘late pupae’). Fruit flies represented under 1% of this second sample. The low percentage of fruit flies is partially due to to the earlier emergence of flies compared with parasites, so most flies remained in the holding container.
Finally, Figure 1 gives the yield in pupal mass from January to June 2010. The yields are measured from pupae enriched in the size range of interest, 1.65-2.26 mm. The large mass of ‘early pupae’ at the beginning of the year indicates a high proportion of fruit flies and unemerged pupae at that time, while adjustments were being made to the new facility.
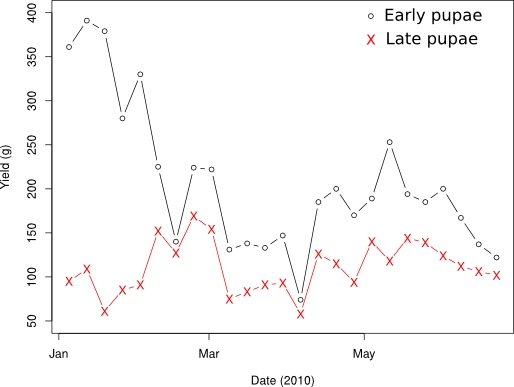
Figure 1. Yield of enriched (pupal diameter 1.65-2.26mm) early (immediately after size selection) and ‘late’ (after holding for one week and removing empty casings) pupae at the ARS-USDA F. ariasnus colony in Hilo, Hawaii, January – June 2010
Discussion
In this protocol and accompanying video article we have described and demonstrated an optimized protocol for rearing F. ariasnus, a parasitoid of Tephritid fruit flies, in a laboratory setting. This protocol has been refined over the years to minimize the amount of labor and specialized equipment needed to maintain a colony. We note that a well established, productive and stable colony of host fruit flies is required for any attempt at rearing parasitoids. In our insectary we use Bactrocera dorsalis as a host fruit fly, but other species have been shown to be competent hosts as well14-17.
Several aspects of F. arisanus colony maintenance remain to be explored. These include the rate of laboratory adaptation in this species10, mechanisms of learning22 and the genetic changes that might occur with adaptation and mechanisms which may be involved in host fruit fly plasticity.
Over the last ten years there have been multiple examples of the spread of Dacine flies throughout the world, particularly those in the genus Bactrocera: B. dorsalis in French Polynesia, B. carambolae in parts of South America, B. invadens in Africa and B. zonata in Africa and Northern Mediterranean.3,23,18 Testing the effectiveness of biological control agents such as F. arisanus against other Bactrocera species should be a high priority, and it is our hope that application of the methods described in this protocol and accompanying video article, will accelerate research on F. arisanus in a wider variety of locations24. Finally, further research using this method as a starting point may also provide important information to the colonization of novel egg parasitoids25,1
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Keith Shigeteni for assistance in the insectary and Natasha Sostrom for help with computer graphics. This work was funded by USDA-ARS. Opinions, findings, conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA. USDA is an equal opportunity provider and employer.
References
- Wang, X. G., Bokonon-Ganta, A. H., Ramadan, M. M., Messing, R. H. Egg-larval opiine parasitoids (Hym., Braconidae) of tephritid fruit fly pests do not attack the flowerhead-feeder Trupanea dubautiae (Dipt., Tephritidae. Journal of Applied Entomology. 128, 716-722 (2004).
- Vargas, R. I. Potential for areawide integrated management of Mediterranean fruit fly (Diptera : Tephritidae) with a braconid parasitoid and a novel bait spray. Journal of Economic Entomology. 94, 817-825 (2001).
- Vargas, R. I., Leblanc, L., Putoa, R., Eitam, A. Impact of introduction of Bactrocera dorsalis (Diptera : Tephritidae) and classical biological control releases of Fopius arisanus (Hymenoptera : Braconidae) on economically important fruit flies in French Polynesia. Journal of Economic Entomology. 100, 670-679 (2007).
- Harris, E. J. Suppression of melon fly (Diptera: Tephritidae) populations with releases of Fopius arisanus and Psyttalia fletcheri (Hymenoptera Braconidae) in North Shore Oahu, HI, USA. Biocontrol. 55, 593-599 (2010).
- Haramoto, F. H. . The Biology of Opius oophilus Fullaway (Hymenoptera: Braconidae) [dissertation]. , (1988).
- Chong, M. Production methods for fruit fly parasites. Proceedings of the Hawaiian Entomological Society. 18, 61-63 (1962).
- Snowball, G. J., Wilson, F., Lukins, R. G. Culture and consignment techiques used for parasites introduced against Queensland fruit fly (Strumeta tryoni (Frogg).). Australian Journal of Agricultural Research. 13, 233-248 (1962).
- Ramadan, M. M., Wong, T. T. Y., Beardsley, J. W. Reproductive Behavior of Biosteres arisanus (Sonan) (Hymenoptera:Braconidae), an Egg-Larval Parasitoid of the Oriental Fruit Fly. Biological Control. 2, 28-34 (1992).
- Ramadan, M. M., Wong, T. T. Y., McInnis, D. Reproductive biology of Biosteres arisanus (Sonan), an egg-larval parasitoid of the oriental fruit fly. Biological Control. 4, 93-100 (1994).
- Harris, E. J., Okamoto, R. Y. A Method for rearing Biosteres arisanus (Hymenoptera, Braconidae) in the laboratory. Journal of Economic Entomology. 84, 417-422 (1991).
- Bautista, R. C., Harris, E. J., Lawrence, P. O. Biology and rearing of the fruit fly parasitoid Biosteres arisanus: clues to insectary propagation. Entomologia Experimentalis Et Applicata. 89, 79-85 (1998).
- Bautista, R. C., Mochizuki, N., Spencer, J. P., Harris, E. J., Ichimura, D. M. Mass-rearing of the tephritid fruit fly parasitoid Fopius arisanus (Hymenoptera : Braconidae). Biological Control. 15, 137-144 (1999).
- Bautista, R. C., Harris, E. J., Vargas, R. I., Jang, E. B. Parasitization of melon fly (Diptera : Tephritidae) by Fopius arisanus and Psyttalia fletcheri (Hymenoptera : Braconidae) and the effect of fruit substrates on host preference by parasitoids. Biological Control. 30, 156-164 (2004).
- Rousse, P., Gourdon, F., Quilici, S. Host specificity of the egg pupal parasitoid Fopius arisanus (Hymenoptera : Braconidae) in La Reunion. Biological Control. 37, 284-290 (2006).
- Quimio, G. M., Walter, G. H. Host preference and host suitability in an egg-pupal fruit fly parasitoid, Fopius arisanus (Sonan) (Hym., Braconidae). Zeitschrift fur Angewandtes Entomologie. 125, 135-140 (2001).
- Calvitti, M., Antonelli, M., Moretti, R., Bautista, R. C. Oviposition response and development of the egg-pupal parasitoid Fopius arisanus on Bactrocera oleae, a tephritid fruit fly pest of olive in the Mediterranean basin. Entomologia Experimentalis Et Applicata. 102, 65-73 (2002).
- Montoya, P., Suarez, A., Lopez, F., Cancino, J. Fopius arisanus oviposition in four Anastrepha fruit fly species of economic importance in Mexico. Biocontrol. 54, 437-444 (2009).
- Vargas, R. I., Shelly, T. E., Leblanc, L., Piñero, J. C. Recent Advances in Methyl Eugenol and Cue-Lure Technologies for Fruit Fly Detection, Monitoring, and Control in Hawaii. Pheromones. 83, 575-595 (2010).
- Tanaka, N., Steiner, L. F., Ohinata, K., Okamoto, R. Low-cost larval rearing medium for mass production of oriental and Mediterranean fruit flies. Journal of Economic Entomology. 62, 967-968 (1969).
- Vargas, R. I. Mass production of tephritid fruit flies. World crop pests. Fruit flies: Their biology, natural enemies and control. 3, 141-151 (1989).
- Spencer, J. P., Mochizuki, N., McInnis, D. O., Liquido, N. J. Mechanical separation of parasitoid sexes based upon size of fruit fly host pupae. , (1996).
- Dukas, R., Duan, J. J. Potential fitness consequences of associative learning in a parasitoid wasp. Behavioral Ecology. 11, 536-543 (2000).
- Drew, R., Tsuruta, K., White, I. A new species of pest fruit fly (Diptera: Tephritidae: Dacinae) from Sri Lanka and Africa. African Entomology. 13, 149-154 (2005).
- Argov, Y., Gazit, Y. Biological control of the Mediterranean fruit fly in Israel: Introduction and establishment of natural enemies. Biological Control. 46, 502-507 (2008).
- Bokonon-Ganta, A. H., Ramadan, M. M., Messing, R. H. Reproductive biology of Fopius ceratitivorus (Hymenoptera : Braconidae), an egg-larval parasitold of the Mediterranean fruit fly, Ceratitis capitata (Diptera : Tephritidae). Biological Control. 41, 361-367 (2007).

