Expansion of Embryonic and Adult Neural Stem Cells by In Utero Electroporation or Viral Stereotaxic Injection
Summary
Controlling the expansion of somatic stem cells is a major factor hampering their study and use in therapy. Here we describe a system to temporally control neural stem cells expansion during development and adulthood, which can be used to increase the number of neurons generated in the mouse brain.
Abstract
Somatic stem cells can divide to generate additional stem cells (expansion) or more differentiated cell types (differentiation), which is fundamental for tissue formation during embryonic development and tissue homeostasis during adulthood 1. Currently, great efforts are invested towards controlling the switch of somatic stem cells from expansion to differentiation because this is thought to be fundamental for developing novel strategies for regenerative medicine 1,2. However, a major challenge in the study and use of somatic stem cell is that their expansion has been proven very difficult to control.
Here we describe a system that allows the control of neural stem/progenitor cell (altogether referred to as NSC) expansion in the mouse embryonic cortex or the adult hippocampus by manipulating the expression of the cdk4/cyclinD1 complex, a major regulator of the G1 phase of the cell cycle and somatic stem cell differentiation 3,4. Specifically, two different approaches are described by which the cdk4/cyclinD1 complex is overexpressed in NSC in vivo. By the first approach, overexpression of the cell cycle regulators is obtained by injecting plasmids encoding for cdk4/cyclinD1 in the lumen of the mouse telencephalon followed by in utero electroporation to deliver them to NSC of the lateral cortex, thus, triggering episomal expression of the transgenes 5-8. By the second approach, highly concentrated HIV-derived viruses are stereotaxically injected in the dentate gyrus of the adult mouse hippocampus, thus, triggering constitutive expression of the cell cycle regulators after integration of the viral construct in the genome of infected cells 9. Both approaches, whose basic principles were already described by other video protocols 10-14, were here optimized to i) reduce tissue damage, ii) target wide portions of very specific brain regions, iii) obtain high numbers of manipulated cells within each region, and iv) trigger high expression levels of the transgenes within each cell. Transient overexpression of the transgenes using the two approaches is obtained by different means i.e. by natural dilution of the electroporated plasmids due to cell division or tamoxifen administration in Cre-expressing NSC infected with viruses carrying cdk4/cyclinD1 flanked by loxP sites, respectively 9,15.
These methods provide a very powerful platform to acutely and tissue-specifically manipulate the expression of any gene in the mouse brain. In particular, by manipulating the expression of the cdk4/cyclinD1 complex, our system allows the temporal control of NSC expansion and their switch to differentiation, thus, ultimately increasing the number of neurons generated in the mammalian brain. Our approach may be critically important for basic research and using somatic stem cells for therapy of the mammalian central nervous system while providing a better understanding of i) stem cell contribution to tissue formation during development, ii) tissue homeostasis during adulthood, iii) the role of adult neurogenesis in cognitive functions, and perhaps, iv) better using somatic stem cells in models of neurodegenerative diseases.
Protocol
Preliminary note
Surgery must be performed by fully trained investigators upon approval from the local authorities for animal welfare. All precautions must be taken to minimize the pain and stress inflicted to the animal including deep anesthesia, administration of postoperative analgesia and, for operations lasting more than 10 min, application of an eye lubricant to prevent corneal dehydration and blindness. Anesthetized mice must be kept constantly warm at 37 °C until full recovery and every surgical tool and solution must be sterile. Although the use of a biological hood is not strictly necessary, the operator must take all reasonable precautions to minimize the possibility of infecting the animal during the operation by wearing a lab coat, mask, and gloves. Similarly, every step concerning the generation and use of viruses must be authorized and must be performed in S2 areas according to the regulations of the local authorities. Finally, a thorough decontamination of all tools and surfaces that could have been in contact with viruses must be performed according to approved facility disinfection practices (for HIV by wiping with 70% Et-OH and/or autoclaving).
Part 1: Expansion of Embryonic NSC
1. In utero electroporation
- After cloning the transgenes (i.e. cdk4/cyclinD1) in pCMS-EGFP (Clontech) or pDSV-mRFPnls vectors 16, purify the plasmids using EndoFree kit and resuspend in sterile PBS to a concentration of 3-5 μg/μl. Soon before surgery, mix the plasmids with 0.05% FastGreen in PBS at a final ratio of ca. 4:4:1 and centrifuge the mixture for 2 min at 16,000 x g to remove all precipitates.
- Load the DNA mixture into a previously pulled borosilicate glass capillary. Pulling parameters using a P-97 pipette puller are: pull: 200; vel: 140; time: 140. Heat is given by a ramp test and depends on the specific lot of capillaries being used. Mount the capillary on the nozzle of the PicoPump that is near to the in utero electroporation platform (Figure 1A) and, under a stereomicroscope, bend its tip and nick it at the inflection point.
- Sterilize all surgery tools in a dry glass bead sterilizer and deeply anesthetize a pregnant mouse at day 12-15 of gestation using an isoflurane vaporizer. Place the animal in supine position on a heating platform set at 37 °C and keep under constant isoflurane administration through a nose cone.
- Shave the skin of the abdomen, disinfect with Betaisodona multiple times, eventually wiping in between with 70% ethanol, and inject buprenorphine (diluted in PBS) subcutaneously at 0.1 mg/kg concentration as pre-emptive analgesic. Using fine scissors, make a 2 cm longitudinal cut of the skin and, subsequently, of the underlying muscular wall to access the peritoneal cavity. Dispense in the peritoneal cavity ca. 2 ml of IUE solution (D-PBS containing 100 U/ml of pen/strep) prewarmed at 37 °C and keep the solution on a heating block.
- Cover the mouse with sterile drap containing a fissure from which the uteri will be removed. Retract the incision using a tungsten retractor, identify the uterus and pull it out holding it with forceps between adjacent embryos (Figure 1B; left) and finally lay it down on the sterile drap. During the whole operation, rinse the uterus with IUE solution to moisturize the organ and body cavity and prevent dehydration of the animal.
- Handle the uterus carefully holding it between thumb and index and turn one embryo until its head is visible and oriented towards the operator. Identify the telencephalic hemispheres and inject one of them from the dorso-lateral side. Release 1-2 μl of the DNA solution using the footswitch of a PicoPump until the ventricle is outlined by FastGreen (Figure 1B; right).
- Place the anode of the electrodes on the injection site and the cathode on the contralateral side (Figure 1C) and deliver 6 pulses of 30 V for 50 ms with 950 ms interval between each pulse using an electroporator generating square-shaped electric fields operated through a footswitch. Avoid placing the electrodes close to the placenta as this may cause hemorrhage and damage the embryo.
- When all embryos are injected and electroporated, place the uterus back into the abdominal cavity and close the muscular walls with a suture string suturing every 3-4 mm. The knots should be snug to the muscle but not too tight to allow a little swelling of the tissue. Finally, close the overlying skin using metal clips. Disinfect the skin with Betaisodona, remove the mouse from the isoflurane mask and transfer it in a housing cage when fully awake. Monitor the recovery of the mouse until locomotor behaviour is normal.
2. Processing of the sample
- One or more days after electroporation, sacrifice the mouse, collect the embryos and identify those correctly electroporated using a fluorescent stereomicroscope (Figure 1D). Dissect the brains on ice-cold PBS, and fix them overnight in 4% PFA (paraformaldehyde in phosphate buffer pH 7.4). Eventually, BrdU can be administered before sacrifice according to different paradigms to investigate cell cycle kinetics and cell proliferation 3.
- The brains are then processed for sectioning and immunostaining for the several markers of radial glia, basal progenitors and neurons (Pax6, Nestin, Tbr2, Tbr1, Tuj1 etc.) to evaluate the effect of the functional transgenes on targeted NSC and their progeny that are identified by the expression of the co-electroporated reporter gene.
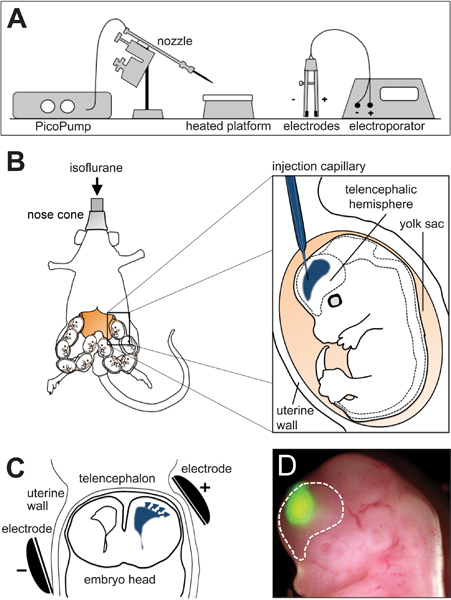
Figure 1. In utero electroporation. (A) Schematic representation of an in utero electroporation platform. (B) Drawings showing the positioning of the pregnant mouse during surgery (right) and injection of the DNA/FastGreen mixture (blue) in the telencephalic hemisphere of a mouse embryo (left). (C) Scheme showing the placement of the electrodes in contact with the uterine walls with the anode (+) adjacent to the site of injection (blue). Arrows indicate the migration of the DNA towards the anode. (D) Picture of a mouse embryo 24 hr after electroporation with GFP plasmids at embryonic day 13. Dashed line demarcates the telencephalic hemisphere. Scheme in A modified from Calegari et al., 2004 17.
Part 2: Expansion of Adult NSC
1. Production of HIV-derived viral particles
- Day 1: plate 5 x 106 293T cells on a 10 cm dish with 8 ml of D-MEM containing 10% of heat inactivated fetal bovine serum and 100 U/ml of pen/strep (culture medium). Use 15 dishes for one viral preparation and culture overnight at 37 °C, 5% CO2.
- Day 2: for every dish, dilute in 1 ml of plain D-MEM 5 μg of each of 3 plasmids encoding for i) VSVG (vesicular stomatitis virus type G) envelope protein, ii) gag/pol (group specific antigen/polymerase), and iii) a polycistronic construct expressing the functional and reporter transgenes (i.e. cdk4/cyclinD1/GFP) previously cloned in a HIV-based transfer vector (p6NST90)9. Mix this solution with 1 ml of D-MEM containing 45 μl of polyethylenimine that has been dissolved beforehand in PBS at 1 mg/ml stock solution, and incubate for 15-30 min at room temperature. In the meanwhile, remove the media from the cells and add 4 ml per dish of fresh D-MEM containing 15% of heat inactivated fetal bovine serum and 100 U/ml of pen/strep. Add the 2 ml of transfection mixture and culture overnight.
- Day 3: add 120 μl of 500 mM Na-butyrate per dish to enhance the expression of the transgenes, mix gently and culture for 6-8 hr after which replace the transfection medium with 5 ml of culture medium.
- Day 4: collect the supernatants (ca. 70 ml total), pass through a 0.22 μm filter and transfer in 2 polyallomer centrifuge tubes (35 x 89 mm). Ultracentrifuge at 110,000 x g for 90 min at 4 °C using a swing rotor. Discard the supernatant, add 6 ml of PBS to each tube and incubate on ice for 1 hr. Resuspend the pellet and transfer it in one polyallomer centrifuge tube (14 x 95 mm). Ultracentrifuge at 110,000 x g for 90 min at 4 °C using a swing rotor. Discard the supernatant, resuspend the pellet in 50 μl of PBS, make 5 μl aliquots and store at -80 °C. Viral titer will be in the range of 108-109 cfu/ml. (Note: viruses are very sensitive to temperature and storage, avoid re-freezing, keep on dry ice during transport and thaw soon before use.)
2. Stereotaxic injection
- Anesthetize a 6-10 weeks old mouse with isoflurane and place the animal in prone position on a stereotaxic frame (Figure 2A) using the ear bars and the palate support to properly fix the head. The animal is kept warm on a heating pad set at 37 °C and under constant isoflurane administration.
- Inject subcutaneously 100 μl of buprenorphine working solution as pre-emptive analgesic, shave a stripe of skin on the head of the mouse, and desinfect as described in 1.4. Using a scalpel, make a ca. 1.5 cm long longitudinal incision on the head skin exposing the skull at the level of the sagittal suture. Retract the skin and gently clean the bone surface with a sterile cotton bud.
- Half-fill a glass capillary (specifications in 1.2) with paraffin oil and mount it on the injector pump inserting the capillary until the second ring (Figure 2B). Move the capillary holder in the x, y and z axis until the tip of the capillary is positioned on the bregma, the conjunction point between the sagittal and lateral sutures (Figure 2C) (to be determined with the help of a stereomicroscope), and re-set x, y, and z values to 0.
- Move the capillary by ±1.6 mm in the medio-lateral axis (x) and -1.9 mm in the anterior-posterior axis (y) and mark the bone using a surgical marker pen. Make a hole of ca. 0.5 mm diameter on the skull using an electric driller paying attention not to damage the brain.
- Put a 5 μl droplet of viral suspension (1 aliquot) on a piece of parafilm placed on the ear bars and immerge the tip of the capillary in the droplet. Suck ca. 3.5 μl of viral suspension using a nanoliter injector set at 55 nl/sec. Execute this step rapidly as the droplet will evaporate.
- Move the capillary to the site of injection and move it down until the tip touches the pial surface. Reset the dorso-ventral axis value (z) to 0. At this point, penetrate the tissue with the capillary for -1.9 mm and release 1.5 μl of viral suspension at a rate of 4 nl/sec. Wait for 2-3 minutes to minimize the backflow of viral suspension trough the capillary track and then retract the capillary. A second injection can be performed in the controlateral hemisphere.
- Suture the skin, disinfect, and allow the animal to recover from anesthesia as described previously for in utero electroporation (1.8).
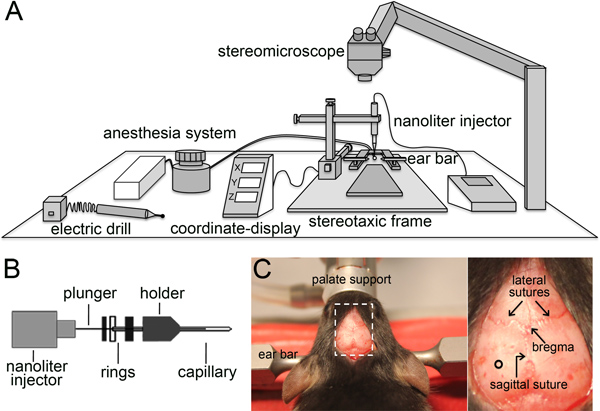
Figure 2. Stereotaxic viral injection. (A and B) Schematic representation of the components needed to perform stereotaxic injection (A) and a disassembled capillary holder showing the insertion of a capillary until the second ring (B). (C) Picture of the mouse head immobilized by the ear bars and palate support with the skin cut to expose the skull (left). The magnification of the dotted box (right) shows the lateral and sagittal sutures, the bregma, and the site of injection (circle).
3. Processing of the sample
- One-to-three weeks post-infection, perfuse the mouse with ca. 100 ml of 4% PFA, dissect the brain and post-fix it overnight in 4% PFA. Eventually, BrdU and/or tamoxifen can be administered before sacrifice according to different paradigms to investigate cell cycle kinetics and to stop the expression of the viral transgenes flanked by loxP sites, respectively 9.
- The brain can then be processed for immunostaining using several markers of stem and progenitors cells and neurons (e.g. Sox2, GFAP, Nestin, Tbr2, doublecortin, etc).
Representative Results
- Overall, in utero electroporation yields 60-80% of electroporated embryos displaying strong expression of the reporter gene in a wide area of the lateral cortex (Figure 3A). Fluorescent proteins can be easily detected on whole mount embryos as soon as 3-6 hours after surgery with the signal lasting for more than one week. Within the targeted area, about 30-50% of cells usually express the transgene(s) with the proportion of cells co-expressing two (or more) co-electroporated transgenes being usually above 90% (Figure 3B, left) 6,8,15. While a minimal level of apoptosis (ca. 2.0-2.5%) could be observed after about 2 hours from electroporation, this value will return to physiological levels (ca. 0.5-1.0%) after about 6 hours (FC, unpublished data). The proportion of embryos or pregnant mice dying due to the surgery is typically negligible (<5%). Co-electroporation with cdk4/cyclinD1 under these conditions followed by 48 hours of development triggers an increase in the expansion of neural progenitor by 30% (Figure 3B, right, and C) 15.
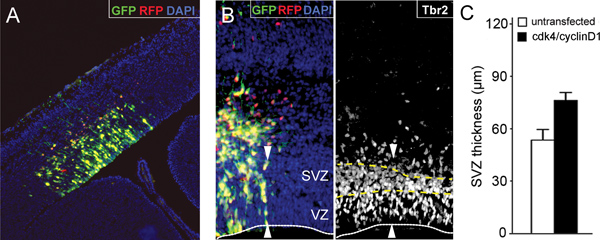
Figure 3. Expansion of neural progenitors by cdk4/cyclinD1 overexpression. (A) Fluorescence picture of a section through the lateral cortex of a mouse embryo 24 hours after electroporation with GFP and RFP plasmids at embryonic day 13. (B) Fluorescent pictures as in A 48 hours after co-electroporation with cdk4/GFP and cyclinD1/RFP plasmids and immunolabeling for the basal progenitor marker Tbr2. Fluorescent reporters with DAPI counterstainig (left) and Tbr2 (right) are shown. Lines indicates the apical surface of the ventricular zone (VZ; white line) and the boundaries of the subventricular zone (SVZ; yellow dashed lines). Note the increased thickness of the SVZ in the electroporated portion of the cortex (white arrowheads) due to an increased generation and expansion of basal progenitors after overexpression of cdk4/cyclinD1. (C) Quantification of the effect shown in B. Bars=SD; p<0.05; n=3. Bar graph in C is taken from Lange et al., 2009 15.
- After viral stereotaxic injection, ca. 80% of operated mice display strong expression of the transgenes throughout the whole dentate gyrus. Constitutive expression of GFP can be easily detected on 40 μm sections as soon as 4 days after surgery. Along the rostral-to-caudal axis of the dentate gyrus, about 10-30% of cells (equivalent to a total of ca. 10,000-30,000 cells) usually express the transgene(s) (Figure 4A) 9. The proportion of mice dying due to the surgery is negligible (<5%). Three week overexpression of cdk4/cyclinD1 under these conditions triggers an increase in NSC by over two-folds (Figure 4B) while decreasing neurogenesis by 70% (Figure 4C) 9.
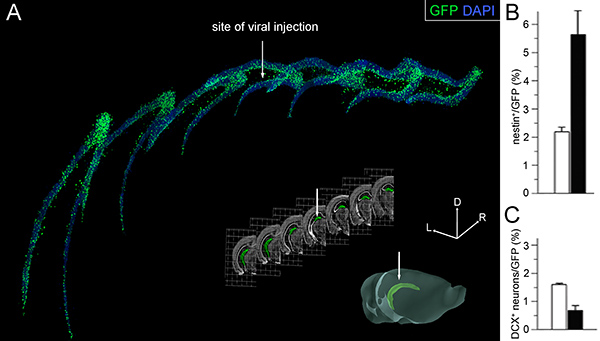
Figure 4. Effects of viral infection. (A) 3D reconstruction of 7 fluorescence pictures taken from 40 μm thick serial coronal sections (collecting 1 every 6) along the rostral-to-caudal axis of the hippocampus. GFP+ infected cells (green) and DAPI nuclear staining (blue) of the dentate gyrus are shown (top). Bright field pictures of the coronal sections corresponding to those shown in A (middle) and 3D representation (bottom) of the whole cerebral hemisphere with the hippocampus highlighted in green pseudocolor (modified from the Allen Brain Atlas; www.brain-map.org). White arrows point the site of injection. Lateral (L), rostral (R), and dorsal (D) axes (x, y, and z stereotaxic coordinates) are indicated (right). (B and C) Proportion of nestin+ NSC (B) and DCX+ newborn neurons (C) within the population of GFP+ infected cells 3 weeks after stereotaxic injection of GFP (white) or cdk4/cyclinD1/GFP (black) viruses. Bars=SD; p<0.005; n=3. Graphs in B and C taken from Artegiani et al., 2011.
Discussion
A critical step for in utero electroporation is the quality and purity of the DNA since its directional migration during delivery of the electric fields depends upon its charge. Protocols for DNA purification that do not include removal of additional charged molecules, such as endotoxins, may lead to the masking of the natural DNA negative charges leading to poor delivery. Clearly, touching the uterine walls with the electrodes as close as possible to the area to be targeted will improve electroporation efficiency. Equally important is the quality of the electrodes used since this is fundamental for the delivery of optimal electric fields. Micro-scratches not even visible by eye and/or organic residues, such as traces of serum, lipids, and so forth, will inevitably accumulate over time leading to electrodes losing their properties. Old and/or dirty electropes are the most likely cause of a sudden drop in electroporation efficiency and, thus, should be replaced as soon as this is noticed. While relatively simple, in utero electroporation typically requires a few weeks of training in order to achieve satisfactorily and reproducible results. This technique is very versatile since it can be applied, virtually, to any organ and tissue of the developing embryo. Certainly, organs containing a cavity, such as the brain, are particularly easy to target and allow for a high proportion of transfected cells due to the ease of injecting a relatively large volume of DNA.
A common problem encountered in setting up stereotaxic viral injection is to have few infected cells and/or to target undesired areas. The first problem is mainly correlated with the injection of low-titer viruses. 293T cells with lower number of passages in culture grow faster and lead to higher viral titers. Using cells older than ca. 20 passages is not recommended and means of transfection with less than 80% efficiency will not ensure a good viral production. Resuspension of the viral pellet is also critical, the viral suspension has to appear homogenous and viral clumps that could occlude the capillary have to be avoided. Viruses are very sensitive to temperature drops and long term storage. For this reasons, aliquots need to be permanently stored at -80 °C and cannot be refrozen. Even under these conditions, a decrease in infectivity could be observed after 6-12 months of storage. In the learning phases, we suggest to test the viral titer of each viral preparation and to do it again after long term storage. The presence of infected cells in undesired areas of the brain can depend on a suboptimal positioning of the mouse head on the stereotaxic frame, which requires some practice. In addition, it is critical not to damage the surface of the brain while opening the hole on the skull because otherwise it will not be possible to reset the 0 correctly on the pial surface, resulting in imprecise injection. The coordinates that we provided in this protocol are referred to C57/BL6 6-10 weeks old females and we suggest optimizing them for different strain/age/sex. Acquisition of this technique may require more time than in utero electroporation due to the longer phases needed to prepare the viruses and analyze the samples. We strongly recommend that viruses are only used after mouse surgery and stereotaxic injection have become reliable and reproducible. The first step to achieve this is to inject cell-permeant DNA dyes, such as Hoechst 33342, in euthanized mice and analyze the brains immediately.
In utero electroporation and stereotaxic viral injection are two powerful systems to acutely and tissue-specifically manipulate gene expression within the CNS during mammalian development and adulthood. Despite their limitation in targeting only a subpopulation of cells, these systems provide an unprecedented speed and ease as compared to more conventional methods, in particular the laborious and time consuming generation of transgenic mice. Yet, despite this similarity, several differences exist between the two techniques that are worth mentioning. First, with regard to the targeting of different cell types, in utero electroporation initially leads to the transfection of stem cells proper (also called radial glial cells) because these are the only cells delimiting the ventricle where the DNA is injected. As a result of cell divisions, plasmids will subsequently be inherited from targeted, mother stem cells to their progenitor and/or neuronal daughter cells leading to the redistribution of transfected cells throughout the cortex as a function of time. In contrast, the use of HIV lentiviruses in the adult brain leads to the simultaneous infection of any cell type including stem cells, progenitors, neurons as well as mature glial cells. Of note, with regard to using HIV lentiviruses as opposed to more commonly used retroviruses is the fact that lentiviral integration would occur independently from the mitotic state of cells, thus allowing the targeting also of adult, quiescent stem cells.
Second, another important difference between electroporation and viral infection is that a transitory, episomal expression is achieved by the former as opposed to the irreversible integration of DNA in the genome of infected cells achieved by the latter. As a result of consecutive cell divisions, dilution of the electroporated plasmids, and degradation of the ectopic cyclinD1 protein, would ensue at each cell cycle. Thus, expansion of NSC during embryonic development was shown to only last for approximately two days 15 while in the adult brain this effect was found to lasts for several months (unpublished results).
Third, since i) cdk4/cyclinD1 has a stronger effect on cells with a longer G1 and ii) committed neural progenitors during development have a longer cell cycle than stem cells while the opposite holds true during adulthood, our two manipulations were found to trigger the expansion, primarily, of neural progenitors during development and stem cells during adulthood. In either case an increase in neurons has been achieved by both methods.
Fourth, viral injection can easily be performed, reliably and reproducibly, in many different regions of the adult brain by simply changing the stereotaxic coordinates. In contrast, precise targeting of certain regions of the central nervous system, such as brain stem, hippocampal formation, or spinal cord, by in utero electroporation might be more difficult to achieve reproducibly. Moreover, while stereotaxic injection can be performed on mice of any age, optimal performance of in utero electroporation is limited from about E11 to E16. Stages prior to E11 would require either the use of an ultrasound backscatter microscope 18,19 or a whole embryo culture system 17,20.
Recently, an increasing interest in basic cell biology of somatic stem cells has been promoted because their study and manipulation are thought to be fundamental towards developing novel cell-based regenerative therapies. In particular for overexpression of cdk4/cyclinD1, our protocol provides the means to transitorily increase the expansion of NSC in vivo (Figure 3B and C and 4B and C) in order to increase the number of neurons generated in the adult brain. We believe that these manipulation may be important in a number of different contexts to better understand the role of NSC in brain development, evolution and homeostasis as well as their contribution to cognitive function or recovery from neural degeneration or injury.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Center for Regenerative Therapies, the Medical Faculty of the TU Dresden, and the DFG Collaborative Research Center SFB655 (subproject A20). BA was supported by a fellowship of the DIGS-BB program, Dresden. We thank Drs. Noriko Osumi, Tadashi Nomura, Dieter Chichung Lie, and Ravi Jagasia for precious advice during the setting up of in utero electroporation and stereotaxic viral injection.
Materials
| Material/equipment | Supplier | Catalog # | |||||||||||||||||||||||||||||||||
| Endofree maxi plasmid kit | Qiagen | 12362 | |||||||||||||||||||||||||||||||||
| FastGreen | Sigma | F7258 | |||||||||||||||||||||||||||||||||
| P-97 Flaming/Brown pipette puller | Sutter Instruments | ||||||||||||||||||||||||||||||||||
| Borosilicate capillaries with filament | Sutter Instruments | BF120-69-10 | |||||||||||||||||||||||||||||||||
| Table top laboratory animal anesthesia system | VetEquip | 901806 | |||||||||||||||||||||||||||||||||
| Isofluran | Baxter | HDG9623 | |||||||||||||||||||||||||||||||||
| Germinator 500 glass bead sterilizer | SouthPointe Surgical | GRM5-1460 | |||||||||||||||||||||||||||||||||
| Iris forceps | WPI | 15917 | |||||||||||||||||||||||||||||||||
| Betaisodona solution | Mundipharma | 1931491 | |||||||||||||||||||||||||||||||||
| Pico Pump | WPI | PV820 | |||||||||||||||||||||||||||||||||
| ECM 830 square wave electroporator | Harvard Apparatus | 450052 | |||||||||||||||||||||||||||||||||
| Round tweezer electrodes | NEPAGENE | CUY650P1 | |||||||||||||||||||||||||||||||||
| Suture material | Ethicon | V493H | |||||||||||||||||||||||||||||||||
| Reflex clip applier for wound closure | WPI | 500345 | |||||||||||||||||||||||||||||||||
| Temgesic injection solution | Essex Pharma AG | ||||||||||||||||||||||||||||||||||
| DMEM w/Glutamax-I | Invitrogen | 31966021 | |||||||||||||||||||||||||||||||||
| Fetal Bovin Serum | Invitrogen | 1027016 | |||||||||||||||||||||||||||||||||
| Penicillin/Streptomicin | Gibco | 15140 | |||||||||||||||||||||||||||||||||
| Tissue culture dish | Falcon | 353003 | |||||||||||||||||||||||||||||||||
| Polyethylenimine 250 ml | Sigma-Aldrich | 40872-7 | |||||||||||||||||||||||||||||||||
| n-Butyric acid, sodium salt | Sigma | B 5887 | |||||||||||||||||||||||||||||||||
| Tube, thinwall, polyallomer | Beckman | 326823 / 331374 | |||||||||||||||||||||||||||||||||
| Model 900 small animal stereotaxic instrument | Kopf Instruments | ||||||||||||||||||||||||||||||||||
| OPMI pico microscope | Zeiss | ||||||||||||||||||||||||||||||||||
| SYS-Micro4 controller and Nanoliter2000 injector | WPI | B203MC4 | |||||||||||||||||||||||||||||||||
| Glass replacement 3.5 nanoliter | WPI | 4878 | |||||||||||||||||||||||||||||||||
| SR drill general application | Foredam | K2272 | |||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
References
- Weissman, I. L. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 100, 157-168 (2000).
- Lindvall, O., Kokaia, Z. Stem cells for the treatment of neurological disorders. Nature. 441, 1094-1096 (2006).
- Salomoni, P., Calegari, F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 5, 332-342 (2010).
- Lange, C., Calegari, F. Cdks and cyclins link G(1) length and differentiation of embryonic, neural and hematopoietic stem cells. Cell Cycle. 9, 1893-1900 (2010).
- Saito, T., Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 240, 237-246 (2001).
- Inoue, T., Krumlauf, R. An impulse to the brain–using in vivo electroporation. Nat. Neurosci. 4, 1156-1158 (2001).
- Tabata, H., Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 10, 865-872 (2001).
- LoTurco, J., Manent, J. B., Sidiqi, F. New and improved tools for in utero electroporation studies of developing cerebral cortex. Cereb. Cortex. , 120-125 (2009).
- Artegiani, B., Lindemann, D., Calegari, F. Overexpression of cdk4 and cyclinD1 triggers greater expansion of neural stem cells in the adult mouse. J. Exp. Med. 208, 937-948 (2011).
- Dixit, R., Lu, F., Cantrup, R., Gruenig, N., Langevin, L. M., Kurrasch, D. M. Efficient Gene Delivery into Multiple CNS Territories Using In Utero Electroporation. J. Vis. Exp. (52), e2957 (2011).
- Walantus, W., Castaneda, D., Elias, L., Kriegstein, A. In Utero Intraventricular Injection and Electroporation of E15 Mouse Embryos. J. Vis. Exp. (6), e239 (2007).
- Matsui, A., Yoshida, A. C., Kubota, M., Ogawa, M., Shimogori, T. Mouse in Utero Electroporation: Controlled Spatiotemporal Gene Transfection. J. Vis. Exp. (54), e3024 (2011).
- Lowery, R. L., Majewska, A. K. Intracranial Injection of Adeno-associated Viral Vectors. J. Vis. Exp. (45), e2140 (2010).
- Lepousez, G., Alonso, M., Wagner, S., Gallarda, B. W., Lledo, P. M. Selective Viral Transduction of Adult-born Olfactory Neurons for Chronic in vivo Optogenetic Stimulation. J. Vis. Exp. (58), e3380 (2011).
- Lange, C., Huttner, W. B., Calegari, F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 5, 320-331 (2009).
- De Pietri Tonelli, D. Single-cell detection of microRNAs in developing vertebrate embryos after acute administration of a dual-fluorescence reporter/sensor plasmid. Biotechniques. 41, 727-732 (2006).
- Calegari, F., Marzesco, A. M., Kittler, R., Buchholz, F., Huttner, W. B. Tissue-specific RNA interference in post-implantation mouse embryos using directional electroporation and whole embryo culture. Differentiation. 72, 92-102 (2004).
- Takahashi, M., Sato, K., Nomura, T., Osumi, N. Manipulating gene expressions by electroporation in the developing brain of mammalian embryos. Differentiation. 70, 155-162 (2002).
- Pierfelice, T. J., Gaiano, N. Ultrasound-Guided Microinjection into the Mouse Forebrain In Utero at E9.5. J. Vis. Exp. (45), e2047 (2010).
- Osumi, N., Inoue, T. G. e. n. e. transfer into cultured mammalian embryos by electroporation. Methods. 24, 35-42 (2001).

