Peering into the Dynamics of Social Interactions: Measuring Play Fighting in Rats
Summary
Play fighting in the rat involves attack and defense of the nape of the neck, which if contacted, is gently nuzzled with the snout. Because the movements of one animal are countered by the actions of its partner, play fighting is a complex, dynamic interaction. This dynamic complexity raises methodological problems about what to score for experimental studies. We present a scoring schema that is sensitive to the correlated nature of the actions performed. Two experiments illustrate how these measurements can be used to detect the effect of brain damage on play fighting even when there is no effect on overall playfulness. That is, the schema presented here is designed to detect and evaluate changes in the content of play following an experimental treatment.
Abstract
Play fighting in the rat involves attack and defense of the nape of the neck, which if contacted, is gently nuzzled with the snout. Because the movements of one animal are countered by the actions of its partner, play fighting is a complex, dynamic interaction. This dynamic complexity raises methodological problems about what to score for experimental studies. We present a scoring schema that is sensitive to the correlated nature of the actions performed. The frequency of play fighting can be measured by counting the number of playful nape attacks occurring per unit time. However, playful defense, as it can only occur in response to attack, is necessarily a contingent measure that is best measured as a percentage (#attacks defended/total # attacks X 100%). How a particular attack is defended against can involve one of several tactics, and these are contingent on defense having taken place; consequently, the type of defense is also best expressed contingently as a percentage. Two experiments illustrate how these measurements can be used to detect the effect of brain damage on play fighting even when there is no effect on overall playfulness. That is, the schema presented here is designed to detect and evaluate changes in the content of play following an experimental treatment.
Introduction
Rats have been the most intensively used animals in the laboratory with which to study play10, 21, 41, and several attempts have been made to develop scoring schemes to measure the effects of experimental treatments on play fighting8, 9, 37, 38, 39. However, while these schemes have had some success in monitoring the frequency of play, they are less suitable as ways to monitor changes in the content of play due to their lack of sensitivity to the dynamics of the interaction13. The main problem is that, during fighting, the use of tactics by one partner is often contingent on the performance of particular tactics by the other2, 15. We have developed a scoring scheme that takes the dynamics of the interaction into account and so is better suited to detect and characterize the subtle changes that occur during play with experimental manipulation16, 20, 26. In this paper, we focus on the measurement of the defensive tactics performed by the recipient of a playful attack.
During serious fighting, animals attack and defend particular body targets, using species-typical weapon systems5. In rats, the primary weapons are the teeth, which are used to bite the flanks and lower dorsum2. Such serious fighting is readily exhibited in a resident-intruder context, in which an unfamiliar adult male is introduced into the cage of a resident adult male2, 15. As in serious fighting, in play fighting, animals also compete to gain some advantage over one another1. Rats, for example, attack and defend the nape of the neck15, which if contacted is gently nuzzled with the snout11, 32. Therefore, it is possible to discern playful attacks from aggressive attacks based not only on the manner of physical interactions (nuzzle vs. bite), but also on the body targets attacked (nape vs. flanks and lower dorsum). In addition, such playful nuzzling should not be confused with social investigation or allogrooming. Social investigation involves sniffing the partner, most frequently on the face and anogenital area. Because these behaviors appear to coincide with playful behaviors, and therefore may be considered aspects of playful interactions, it has been shown that these social interactions precede play behavior. Furthermore, changes in the motivation to engage in social investigation are independent of comparable changes in play9. There are several features that distinguish allogrooming from play20. First, allogrooming usually occurs when the rats huddle together following a protracted period of playing. Second, while playful attack is centered on the nape, allogrooming can involve contact with many parts of the partner’s body, although it sometimes is focused on the nape. Third, this grooming involves gentle nibbling and pulling of the partner’s fur with the teeth. Fourth, unlike allogrooming, most playful attacks to the nape are defended by the recipient, which leads to complex sequences of wrestling.
The main focus of this paper is to show what may be fruitfully measured during those complex sequences of play that can include wrestling. As shown below, the various tactics used by the defender to protect its nape lead to different possible outcomes. These outcomes, such as wrestling on the ground, boxing or chasing, have in some studies been measured as independent behavior patterns, but we have found that, in the majority of cases, these outcomes depend on the defensive tactic adopted by the recipient of a nape attack15. For example, evasive tactics often lead to chases and rolling over onto the back leads to wrestling. Thus, outcome based measurements, such as boxing and wrestling, are actually composites arising from the actions of both participants. Using a dynamic approach, our framework provides a means of scoring moments in the interaction when the actions measured are attributable to only one of the participants28, an approach that will be more evident to the reader once the examples of how the measuring framework is used are presented.
As shown in Figure 1, the rat on the left is seen approaching another from the rear (a) and then pouncing toward the nape of its neck (b). However, before contact is made, the defender rotates around the longitudinal axis of its body (c) to face its attacker (d). As the attacker moves forward, the defender is pushed onto its side (e), but then rolls over onto its back as the attacker continues to reach for its nape (f-h). From the supine position, the defender launches an attack toward its partner’s nape (i), but is blocked by its partner’s hind foot (j, k). Following another attempt to gain access to its partner’s nape, the rat on top is pushed off (l) by the supine animal’s hind feet (m), which thus enables the original defender to regain its footing (n) and lunge to attack its partner’s nape (o).
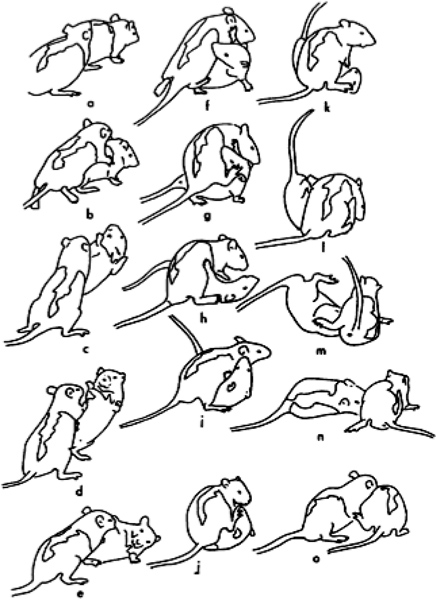
Figure 1. A pair of juvenile male rats is shown attacking and defending the nape during a play fight. From Pellis & Pellis15; reprinted with permission of John Wiley & Sons.
Protocol
1. Equipment and Room Set Up
- The play box apparatus is a clear, Plexiglas box measuring 50 cm X 50 cm X 50 cm with 1-2 cm of standard corncob bedding.
- All filming is conducted in the dark via a handheld camcorder under the night-shot option.
- The camcorder is placed at a 45° angle facing downwards towards the play box on a tripod. All four corners must be visible in the shot to obtain a full view of the apparatus.
2. Methodology
- Animals are weaned between postnatal days 21-23. Depending on the experimental design, animals can be housed together in groups of 2-4 in the required male and female combinations.
- Play testing occurs during an animal’s light cycle, although testing occurs in a dark room. The testing room must be dark prior to the entrance of the animals.
- Groups housed together are habituated for three, consecutive 30-min, sessions directly prior to testing following the same sequence as if being tested (see 2.5).
- Following habituation, pairs are individually isolated 24 hr prior to testing to maximize playful interactions (see 2.5).
- Animals are transported into a dark testing room and are then individually placed in the play apparatus and tested as pairs for 10 min. Subjects are then removed and placed together in their home cage.
- Following each play session, bedding is discarded and the play apparatus is thoroughly cleaned using the antiseptic Virkon (or its equivalent). This is a superior agent for cleaning the rat odors from the previous pair, as it does not leave the strong and persistent smell of alcohol wipes or sprays. Cover-spray may also be used to mask any scents given off by the subjects. New bedding is then added.
- Repeat steps (2.4-2.6) for additional trials.
3. Behavioral Analysis: Assessing the Content of Play
First, the distinctive targets competed over by rats in both serious fighting and play fighting make this species particularly valuable as a research subject for studying play, as escalation from playful to serious fighting can be unambiguously detected by a shift in body targets35. Second, the number of attacks launched against the nape of a partner can be measured. Third, given that defense is contingent on attack, the relative defensiveness of the recipient of attacks can be measured as the proportion of attacks defended. Fourth, when defending against a nape attack, the recipient can use one of several tactics to protect or extricate their nape from their partner’s snout15, 25, 26. These tactics fall into two main categories.
- In evasion, the defender can walk, run, leap or dodge away (Figure 2a), and, by doing so, it not only moves its nape away from its attacker, but also ends up facing away from its attacker (Video of evasive defense).
- In facing defense, the defender turns to face its attacker, thus not only withdrawing its nape away, but also juxtaposing its teeth between its nape and the attacker’s snout. Facing defense falls into two main categories: rotation around the longitudinal axis of the body and rotation, horizontally, around a vertical axis (usually between the rump and mid-body, although this can differ between the sexes27). Further, rotation around the longitudinal axis of the body can take one of two forms.
- Complete rotation involves the rat turning from a standing position to fully supine (Figure 2B). Partial rotation involves the rat turning its forequarters, but leaving one or both of its hind paws in contact with the ground (Figure 2C, Video to show difference between full rotation and partial rotation).
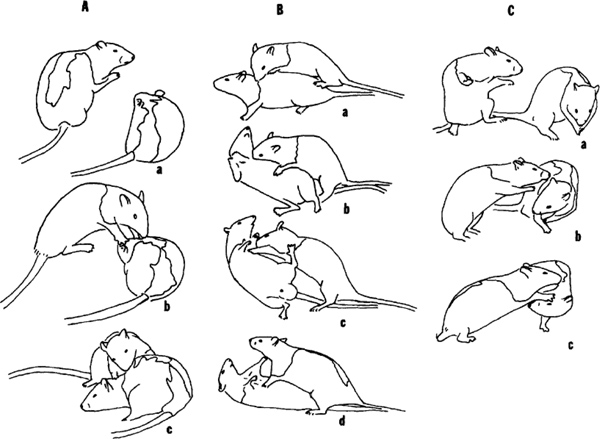
Figure 2. Three types of defense in response to a playful nape attack are shown in 61 days old male rats (A-C). A. Evasion. Following the attacker’s lunge at the nape (a, b), the defender swerves away from its attacker (c). B. Complete rotation. A nape contact from behind (a) leads the defender to rotate (b, c) until it is lying supine and blocking the attacker with its outstretched paws (d). C. Partial rotation. An attacker’s lunge to its partner’s nape from the side (a, b) is followed by a rotation of the head, neck and shoulders by the defender, so withdrawing its nape from its attacker’s snout, while maintaining support on the ground with its hind feet (c). From Pellis et al. 26; reprinted with permission of S. Karger AG, Basel.
- Horizontal rotation around the vertical axis requires the defender to move its rump away from its attacker while moving its head toward its partner (Figure 3, Video of horizontal rotation).
- Following its immediate rotation around either its vertical axis or partially around its longitudinal axis, the defender can then rear and push against its partner, showing that the outcome, when using either of these two tactics, can be similar. Confounding tactics with outcomes can be even more striking when considering complete and partial rotations. When a defender executes a complete rotation, the movement is quick and fluid – if videotaped on a standard 30 fps recording, a rotation to fully supine takes 2-3 frames and the movement is continuous (Video).
- A partial rotation can also end in a fully supine position, but this typically involves the concerted actions of the attacker. In this case, the defender can partially rotate its forequarters and keep its body horizontal or even touching the ground, but the attacker can then press its body weight onto the defender. With persistent pressing and reaching for the nape by the attacker, the defender rotates more and more, until fully supine. Such a ‘forced’ supine occurs in a staccato manner, with incremental rotation of the body, and can take substantially more than 3 video frames (Video showing a partial rotation leading to the defender adopting a supine position).
Given that most studies are interested in the effects of particular manipulations on subjects, it is best to score the tactic attempted by the subject rather than the eventual outcome that may be forced by its partner’s actions. This judgment can be made by examining the movements of the defender within the first 2-3 frames of the commencement of a defensive action (Video highlighting the first 2-3 frames, frame-by-frame).
- Playful attacks can be measured when the snout of one rat either contacts or comes within 1-2 cm of its partner’s nape (Video showing a playful attack compared to non-playful movements near partner rat).
- As noted above, the defensive tactic of the recipient can be categorized in the first few frames following an attack. A new sequence begins with a new attack (Video showing extended playful sessions compared to separate playful attacks).
- During an extended playful wrestle, such as is shown in Figure 1, rats can counterattack once they have successfully defended themselves, but these types of attacks are more difficult to score, especially in regard to the partner’s defensive reaction. Because counterattacks are highly correlated with the frequency of initiating attacks16, these more complex phases of the interactions need not be scored unless there is good reason for doing so23. The scoring of initiating attacks and the tactics employed to defend against such attacks suffice to capture the relative differences in playfulness and defensive behavior of rats in different experimental conditions26, 29.
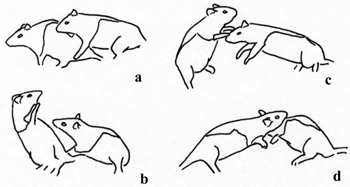
Figure 3. Facing defense involves a defender turning to face its attacker while pivoting on its hind legs in the horizontal plane around a vertical axis (a, b). From this position, the defender can block access to its nape (c) and launch a counterattack (d). From Pellis et al.25; adapted and reprinted with permission from APA.
Since most playful interactions start with a playful attack, the absolute number of such attacks can be measured within the time period of a test trial. The optimal size of the trial for our studies has been 10 min following 24 hr of social isolation15, 16. Brief periods of social isolation increases the motivation to engage in social play while not having a similar effect on other social behavior such as social investigation9. Although any amount of isolation has this effect, the impact of isolation on play reaches an asymptote around 24 hr27. While trials of 5 min can detect such isolation-induced changes, they are too short to detect subtle differences such as those between the sexes due to a ceiling effect – in this example, the greater propensity for males to play mostly emerges after the first 5 min. Even so, by 15 min, the animals appear fatigued by the vigorous play and consequently, trials lasting 10 min are a good compromise between economy of protocol and the ability to detect experimental effects15, 16, 27. Whether tested in the light or dark phase of the rats’ daily cycle, our experience is that the amount of play increases if the rats are tested under red light instead of white light15, 16. With the advent of camcorders with night shot capability, filming in the dark is possible, and this also increases the amount of play performed in test trials29.
- Given that defense is contingent on an attack occurring, defensiveness should be scored as a proportion of attacks leading to defense. Similarly, given that any given type of defense is contingent on a defense occurring, scoring types of defense should also be in terms of proportions (e.g., of the defenses that occurred, X% were complete rotations). Scoring defense as an absolute measure can be misleading. For example, a low frequency of complete rotations could arise because of low rates of attack or because the subjects in that particular experimental condition used the complete rotation infrequently even if they had been attacked just as frequently as the animals in the control condition.
- Thus, when scoring play, there is a sequential analysis: how many attacks are launched by the subjects (number/unit time), how many attacks are defended against (i.e., probability of defense), and when defending, what is the likelihood of using the different tactics of defense (%tactics).
4. Behavioral Analysis: Using the Content of Play to Assess Play Partner Relationships
With the onset of puberty, there is an overall decline in the amount of play fighting8, 40. This is because they decrease the frequency of their launching of nape attacks, but maintain the same, high probability of defense17. Among male rats, the pattern of play is modified by the development of dominance relationships that coalesce around puberty37. This is reflected in the types of defensive tactics used.
- In males, with the onset of puberty, there is a relative decline in their use of the complete rotation tactic (Different Video of Full Rotation as shown in above parts) and an increase in their use of the partial rotation tactic (Different Video of Partial Rotation as show in above parts), with the other tactics remaining at about the same frequency at all ages16.
- However, in subordinate males, it is dependent on their partner as to which of these tactics they will use. When playing with another subordinate male or a female, a subordinate male rat is most likely to use the partial rotation tactic when attacked, but when playing with the dominant male in the colony, it is more likely to use the complete rotation tactic when attacked18, 19, 24, 36. Thus, with the onset of dominance relationships, the relative use of tactics is a good marker to monitor changes in an experimental subordinate rat’s social relationships12.
Representative Results
Two studies illustrate how the multi-faceted scoring scheme that we have developed can be used to diagnose the effects of experimental manipulations on the play fighting of rats. The first shows that the method can be used to pinpoint the specific component of play changed by an experimental manipulation and the second shows how the method can be used to measure changes in the relationships following an experimental manipulation.
(1) The effects of neonatal decortication
Complete removal of the cortex can lead to adult rats that can function relatively normally in laboratory environments42, however, given the presumed role of large brains in promoting play in mammals3, we wanted to test whether play is reduced in rats without a cortex. The play fighting of juvenile and adult rats decorticated at birth, were compared with sham-treated controls26.
A commonly used measure for play, ‘pinning’9, essentially involves the subject positioned supine with its partner standing on top (see frame g in Figure 1). When the effects of decortication were measured using pinning, there was about a 50% reduction in this measure in the juvenile period10, 26. Taken by itself, such a measure could lead to the interpretation that decorticates have reduced motivation to engage in play. However, measuring nape contacts10, or in our conception, nape attacks26, there is no difference in the frequency of initiating play between intact and decorticate rats. Thus, decorticates appear to be normally motivated to engage in play, a conclusion supported by the finding that the probability of defense does not differ from that of intact rats26. The problem remains as to why the frequency of being pinned was halved in decorticates, and the answer was that, when defending themselves, there was a striking difference – decorticates behaved more like adults in using the partial rotation more often than the complete rotation (Table 1). Thus, decorticates infrequently adopted a defensive strategy – complete rotation – that is the most common route by which pins arise.
Subsequent analyses showed that damage limited to the motor cortex was sufficient to replicate this effect on juvenile rats6. The column in Table 1 for the control rats shows the typical values for these measures when using Long-Evans hooded rats. Researchers should be attentive to wide deviations from these ‘typical’ values for some other strains4, 27, 30, 31, 33, 34. Therefore, a baseline value for untreated pairs from the respective strain used should be established before commencing an experimental procedure.
| Play measures | Intact | Decorticate | Difference (t-test)# |
| Pins | 40+ | 18 | * |
| Playful attacks | 53 | 49 | ns |
| Probability of defense | .91 | .93 | ns |
| Evasion (%) | 20 | 25 | ns |
| Complete rotation (%) | 60 | 20 | * |
| Partial rotation (%) | 20 | 60 | * |
+Only mean values are shown for illustrative purposes. See original and other citations to gain a sense of the variance in these measures.
#Significance is indicated by * which in these cases is at the p < 0.05 or lower. Non-significant differences are indicated by ns.
Table 1. Various measures of play fighting are compared for intact and decorticate juvenile male rats. Adapted from Pellis et al.26
(2) The prefrontal cortex and the modulation of play
In one of the conditions tested in the decortication experiment, the rats were housed together in groups of four males, two with the cortex removed and two controls. As adults, decorticates seemed to fail to modify their play when confronted with the dominant member of the group. The orbital frontal cortex (OFC) was tested as a likely candidate structure in such modulation, given its role in regulating social behavior generally7. Groups of three adults were formed – two males and a female, with the female having her fallopian tubes tied to avoid the confounding influence of pregnancy. After two weeks, the animals were tested in paired trials to determine which of the males in each triad was the subordinate one (see Smith et al.35, for criteria to determine dominance). The subordinates in some triads had their OFC removed and, in other triads, the subordinates were sham-treated. Then, after recovery from surgery and two weeks to ensure the re-establishment of social relationships, the rats from each triad were re-tested in pairings29. There was no significant difference either before or after surgery in the frequency of playful attacks, the probability of defense or in the use of facing defense between the subordinates in the experimental and control conditions. However, the ability of the OFC rats to play differently with the two types of partners was compromised.
To assess this, the probability of the use of complete rotation with the female was subtracted from that occurring when playing with the dominant male and was then converted to a percentage – this type of defense should be high when the subordinate male is attacked by the dominant male and low when attacked by the female. This yields an asymmetry index, so that the closer the score is to 100%, the more asymmetrical, and the closer to 0%, the more symmetrical. Following the lesion and the re-establishment of social relationships, the controls continued to show a high degree of asymmetry, whereas the OFC damaged rats showed virtually no difference in their pattern of defense when attacked by the dominant male or by the female (Figure 4). That is, even though the OFC damaged subordinates played just as much as the controls, they were no longer able to discriminate between their pair mates, supporting the hypothesis that partner-related modulation of play and other social behaviors is mediated by the OFC29.
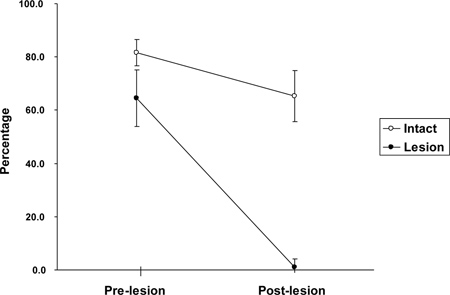
Figure 4. Change in the asymmetry shown by the subordinate male rats when playing with the dominant colony male compared with the colony female. The measure shows the percentage difference in using the complete rotation when interacting with these two types of partners (see text). Lesions of the OFC abolish the subordinates’ ability to play differentially with its cage mates. Sham-treated subordinate males (anesthetized, skull opened, but not given a lesion) continue to show a strong asymmetry after the surgery. From Pellis et al. (2006); reprinted with permission from APA.
Discussion
Play fighting is a complex, dynamic interaction in which the participants continually influence each other’s behavior. As a consequence, a scoring scheme that is sensitive to those dynamics and the contingent nature of the actions performed is needed. The scheme outlined in this paper has proven successful in comparing across sexes, individual differences, through development, strain differences and for experimental treatments4, 14, 16, 20, 24, 29, 30, 31, 32, 32, 36. Moreover, the scheme presented can be used as a skeleton on which to build further refinements. For example, the distance at which rats respond to the approach of an attacker can influence the type and the success of the tactics executed25. Similarly, such differences in opportunity can be countered methodologically by scoring the defensive options only in highly specific and comparable contexts28. In the case of play fighting, this can be when the attacker is approaching the neck from a perpendicular angle and the recipient is at least one body length away from a wall, thus removing the confounds that could arise from opportunity rather than capacity22. Thus, the currently proposed framework for scoring the actions performed during play can be thought of as a skeleton, upon which additional measurements can be incorporated as needed. In some situations, the current framework is likely to be inadequate if used alone. For example, a recent analysis of juvenile play in wild-type rats compared to Long-Evans rats showed that, because defensive actions began at a bigger inter-animal distance than in the wild rats, measuring nape attacks alone underestimated the measure of play. Scoring outcome measures such as chasing and boxing was important to obtain a more balanced picture of the differences in the play of these two strains (Himmler et al., work in progress). Therefore, although the currently proposed framework may have limitations when used as the sole method of studying play, modifications can be introduced in a manner that meets specific research goals and does so while retaining the guiding principle of the contingent and dynamic nature of such interactions13.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the Harry Frank Guggenheim Foundation and the Natural Science and Engineering Research Council of Canada for grants to SMP that supported the research leading to the development of these methods.
Materials
| Equipment | Comments (optional) |
| Sony DCR-DVD103 Camcorder | Optical Zoom- 20x, Night shot *See Below |
| Play Apparatus | 50 X 50 X 50 cm Plexiglas |
* Any camcorder will work for recording, although an optical zoom of 20x or above is desired for optimal zoom. |
References
- Aldis, O. . Play Fighting. , (1975).
- Blanchard, R. J., Blanchard, D. C., Takahashi, T., Kelley, M. J. Attack and defensive behaviour in the albino rat. Anim. Behav. 25, 622-634 (1977).
- Fagen, R. A. . Animal Play Behavior. , (1981).
- Field, E. F., Watson, N. V., Whishaw, I. Q., Pellis, S. M. Play-fighting in androgen-insensitive tfm rats: Evidence that androgen receptors are critical for the development of adult playful defense but not playful attack. Dev. Psychobiol. 48, 111-120 (2006).
- Geist, V., Krames, L., Pliner, P., Alloway, T. On weapons, combat and ecology. Advances in the Study of Communication and Affect. 4, 1-30 (1978).
- Kamitakahara, H., Monfils, M. -. H., Forgie, M. L., Kolb, B., Pellis, S. M. The modulation of play fighting in rats: Role of the motor cortex. Behav. Neurosci. 121, 164-176 (2007).
- Kolb, B., Kolb, B., Tees, R. C. Prefrontal cortex. The Cerebral Cortex of the Rat. , 437-458 (1990).
- Meaney, M. J., Stewart, J. A descriptive study of social development in the rat (Rattus norvegicus. Anim. Behav. 29, 34-45 (1981).
- Panksepp, J. The ontogeny of play in rats. Dev. Psychobiol. 14, 327-332 (1981).
- Panksepp, J., Normansell, L., Cox, J. F., Siviy, S. M. Effects of neonatal decortication on the social play of juvenile rats. Physiology and Behavior. 56, 429-443 (1994).
- Pellis, S. M. Agonistic versus amicable targets of attack and defense: Consequences for the origin, function and descriptive classification of play-fighting. Aggress. Behav. 14, 85-104 (1988).
- Pellis, S. M., Bekoff, M., Allen, C., Burghardt, G. M. Keeping in touch: Play fighting and social knowledge. The Cognitive Animal: Empirical and Theoretical Perspectives on Animal Cognition. , 421-427 (2002).
- Pellis, S. M., Bell, H. C. Closing the circle between perceptions and behavior: A cybernetic view of behavior and its consequences for studying motivation and development. Dev. Cog. Neurosci. 1, 404-413 (2011).
- Pellis, S. M., McKenna, M. M. Intrinsic and extrinsic influences on play fighting in rats: Effects of dominance, partner’s playfulness, temperament and neonatal exposure to testosterone propionate. Behav. Brain Res. 50, 135-145 (1992).
- Pellis, S. M., Pellis, V. C. Play-fighting differs from serious fighting in both target of attack and tactics of fighting in the laboratory rat Rattus norvegicus. Aggress. Behav. 13, 227-242 (1987).
- Pellis, S. M., Pellis, V. C. Differential rates of attack, defense and counterattack during the developmental decrease in play fighting by male and female rats. Dev. Psychobiol. 23, 215-231 (1990).
- Pellis, S. M., Pellis, V. C. Attack and defense during play fighting appear to be motivationally independent behaviors in muroid rodents. Psych. Rec. 41, 175-184 (1991).
- Pellis, S. M., Pellis, V. C. Role reversal changes during the ontogeny of play fighting in male rats: Attack versus defense. Aggress. Behav. 17, 179-189 (1991).
- Pellis, S. M., Pellis, V. C. Juvenilized play fighting in subordinate male rats. Aggress. Behav. 18, 449-457 (1992).
- Pellis, S. M., Pellis, V. C. The pre-juvenile onset of play fighting in rats (Rattus norvegicus. Dev. Psychobiol. 31, 193-205 (1997).
- Pellis, S. M., Pellis, V. C. The play fighting of rats in comparative perspective: A schema for neurobehavioral analyses. Neurosci. Biobehav. Rev. 23, 87-101 (1998).
- Pellis, S. M., Pellis, V. C. The Playful Brain. Venturing to the Limits of Neuroscience. , (2009).
- Pellis, S. M., Pellis, V. C., Dewsbury, D. A. Different levels of complexity in the playfighting by muroid rodents appear to result from different levels of intensity of attack and defense. Aggress. Behav. 15, 297-310 (1989).
- Pellis, S. M., Pellis, V. C., McKenna, M. M. Some subordinates are more equal than others: Play fighting amongst adult subordinate male rats. Aggress. Behav. 19, 385-393 (1993).
- Pellis, S. M., Pellis, V. C., McKenna, M. M. A feminine dimension in the play fighting of rats (Rattus norvegicus) and its defeminization neonatally by androgens. J. Comp. Psych. 108, 68-73 (1994).
- Pellis, S. M., Pellis, V. C., Whishaw, I. Q. The role of the cortex in play fighting by rats: Developmental and evolutionary implications. Brain Behav. Evol. 39, 270-284 (1992).
- Pellis, S. M., Field, E. F., Smith, L. K., Pellis, V. C. Multiple differences in the play fighting of male and female rats. Implications for the causes and functions of play. Neurosci. Biobehav. Rev. 21, 105-120 (1997).
- Pellis, S. M., Pellis, V. C., Pierce, J. D., Dewsbury, D. A. Disentangling the contribution of the attacker from that of the defender in the differences in the intraspecific fighting of two species of voles. Aggress. Behav. 18, 425-435 (1992).
- Pellis, S. M., Hastings, E., Shimizu, T., Kamitakahara, H., Komorowska, J., Forgie, M. L., Kolb, B. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and non-playful social contexts. Behav. Neurosci. 120, 72-84 (2006).
- Reinhart, C. J., Pellis, S. M., McIntyre, D. C. The development of play fighting in kindling-prone (FAST) and kindling-resistant (SLOW) rats: How does the retention of phenotypic juvenility affect the complexity of play. Dev. Psychobiol. 45, 83-92 (2004).
- Reinhart, C. J., Metz, G., Pellis, S. M., McIntyre, D. C. Play fighting between kindling-prone (FAST) and kindling-resistant (SLOW) rats. J. Comp. Psych. 120, 19-30 (2006).
- Siviy, S. M., Panksepp, J. Sensory modulation of juvenile play in rats. Dev. Psychobiol. 20, 39-55 (1987).
- Siviy, S. M., Baliko, C. N., Bowers, K. S. Rough-and-tumble play behavior in Fischer-344 and buffalo rats: Effects of social isolation. Physiol. Behav. 61, 597-602 (1997).
- Siviy, S. M., Love, N. J., DeCicco, B. M., Giordano, S. B., Seifert, T. L. The relative playfulness of juvenile Lewis and Fischer-344 rats. Physiol. Behav. 80, 385-394 (2003).
- Smith, L. K., Fantella, S. -. L., Pellis, S. M. Playful defensive responses in adult male rats depend upon the status of the unfamiliar opponent. Aggress. Behav. 25, 141-152 (1999).
- Smith, L. K., Forgie, M. L., Pellis, S. M. Mechanisms underlying the absence of the pubertal shift in the playful defense of female rats. Dev. Psychobiol. 33, 147-156 (1998).
- Takahashi, L. K., Lore, R. K. Play fighting and the development of agonistic behavior in male and female rats. Aggress. Behav. 9, 217-227 (1983).
- Taylor, G. T. Fighting in juvenile rats and the ontogeny of agonistic behavior. J. Comp. Physiol. Psych. 94, 953-961 (1980).
- Thor, D. H., Holloway, W. R. Play solicitation behavior in juvenile male and female rats. Anim. Learn. Behav. 11, 173-178 (1983).
- Thor, D. H., Holloway, W. R. Developmental analysis of social play behavior in juvenile rats. Bull. Psychon. Soc. 22, 587-590 (1984).
- Vanderschuren, L. J. M. J., Niesink, R. J. M., van Ree, J. M. The neurobiology of play behavior in rats. Neurosci. Biobehav. Rev. 21, 309-326 (1997).
- Whishaw, I. Q., Kolb, B., Tees, R. C. The decorticate rat. Cerebral cortex of the rat. , 239-268 (1990).

