Vertical T-maze Choice Assay for Arthropod Response to Odorants
Summary
A vertical, T-maze olfactometer is described for assaying the behavioral response of arthropods. The olfactometer allows the experimenter to measure choices performed by test subjects when subjected to two potential odor fields. Both attraction to and repulsion from odorants can be measured with this device.
Abstract
Given the economic importance of insects and arachnids as pests of agricultural crops, urban environments or as vectors of plant and human diseases, various technologies are being developed as control tools. A subset of these tools focuses on modifying the behavior of arthropods by attraction or repulsion. Therefore, arthropods are often the focus of behavioral investigations. Various tools have been developed to measure arthropod behavior, including wind tunnels, flight mills, servospheres, and various types of olfactometers. The purpose of these tools is to measure insect or arachnid response to visual or more often olfactory cues. The vertical T-maze oflactometer described here measures choices performed by insects in response to attractants or repellents. It is a high throughput assay device that takes advantage of the positive phototaxis (attraction to light) and negative geotaxis (tendency to walk or fly upward) exhibited by many arthropods. The olfactometer consists of a 30 cm glass tube that is divided in half with a Teflon strip forming a T-maze. Each half serves as an arm of the olfactometer enabling the test subjects to make a choice between two potential odor fields in assays involving attractants. In assays involving repellents, lack of normal response to known attractants can also be measured as a third variable.
Introduction
Arthropods (including insects and arachnids) are often defined as pests, because they either compete with human consumption in agricultural or urban settings or transmit causal pathogens of disease1. A large amount of investment is made into development of control tools for such pests. These tools can vary widely and significant progress has been made in developing biorational tools that either modify or exploit the natural behavior of pests with the use of behavioral attractants or repellents2,3.
Arthropod pests often rely on olfactory signals or cues for mate and/or host location4. Therefore discovery of effective attractants or repellents for pests can often lead to development of control tools. This process of discovery involves several steps. First, the natural behavior of the test subject must be understood. For example, one must ascertain whether one sex of a given species attracts the opposite sex for mate location. If so, one must determine whether this attraction is mediated by a chemical and where that chemical is produced. Further steps include determining when the chemical is produced and its eventual identification. Throughout this procedure, the test subject’s (insect or mite) behavioral response to odorants must be tested. Initially, the behavior of the test subject will be tested to the natural material. For example, a male moth’s response may be tested to that of the extract of its female conspecific’s pheromone gland. Alternatively, an herbivore’s response to the natural odors of its host plant may be tested. While these investigations often have a practical focus on development of management tools5,6, they can also be applied to research that addresses more fundamental questions, such as speciation7. Subsequent steps involve testing the arthropod’s response to synthetic versions of identified natural products.
Understanding how arthropods respond to chemicals requires the development of appropriate tools to measure their behavior. The data generated by such tools will depend on how well the investigator is able to measure natural behaviors in a controlled setting. Over six decades ago, sustained flight olfactometers and tunnels were developed to measure insect response to odorants8,9. Olfactometers vary significantly in design and are often tailored to the specific insect or problem being investigated. The goal of this article is to describe the design and use of a vertically oriented, T-maze olfactometer for measuring insect response to odorants (Figure 1). The olfactometer’s vertical orientation takes advantage of the negative geotaxis (tendency to move up) exhibited by many insects. A light source positioned directly above the olfactometer takes advantage of the positive phototaxis (tendency to move toward source of light) exhibited by many insects. The olfactometer is particularly useful for hemipteran insects that tend not to exhibit movement readily in horizontally-oriented olfactometers and that are heavily influenced by light intensity with respect to orientation. However, this device can be used for a wide range of flying and crawling insects, as well as arachnids. The olfactometer can be employed to evaluate both attractants and repellents. In the case of attractants, the bifurcated odor field (Figure 1) is established with the putative attractant released on one side versus clean air released on the opposite side. A choice is recorded when the insect released at the base of the olfactometer chooses one of the two odor fields. In the case of repellents, a third behavior is possible. In addition to upward movement into either of the two odor fields, lack of upward movement may be recorded indicating a non-responsive stimulus or repelling stimulus from either odor fields.
Protocol
- Assembly of a custom-designed, two-port divided T-olfactometer previously described in Mann et al.10 is shown in Figure 1. The olfactometer consists of a 30 cm glass tube that is bifurcated into two separate ports with a Teflon strip forming a T-maze. Each half is analogous to an arm of a 2-choice style olfactometer enabling the test subject to choose between two potential odor fields.
- Assembly and subsequent assays should take place within a temperature-controlled room or environmental chamber.
- Mount glass T-maze olfactometer vertically as shown in Figure 1 and position it under a fluorescent 900 lux light bulb.
- For optimal light diffusion, the assembly should be mounted within a 1.0 x 0.6 x 0.6 m opaque container to maximize uniform light diffusion.
- Connect the olfactometer arms to odor sources that will be housed in solid-phase micro-extraction holding/odor source chamber (SPMEC) (ARS, Inc., Gainesville, FL) through Teflon;-glass tube connectors. Each SPMEC is comprised of a straight glass tube (17.5 cm long x 3.5 cm wide) supported with an inlet and outlet valve for incoming and outgoing air streams (Figure 1).
- Connect the air delivery system to an external source of humidified and carbon-purified air that is under constant pressure (the air delivery system has both an internal carbon filter for air purification and an air bubbler for humidification if external sources are unavailable).
- Dissolve desired chemical samples within appropriate diluent (solvent) and pipette onto a slow release substrate such as a cotton wick (Petty John Packaging, Inc., Concord, NC). The control treatment should consist of a cotton wick impregnated with solvent only.
- To prevent significant contamination of the glassware, envelop the treated and control cotton wicks in laboratory tissue (Kimwipes, Kimberly-Clark, Roswell, GA) and place into the two SPMEC arms (ARS, Inc., Gainesville, FL). In the case of live plant samples, a guillotine volatile collection chamber can be attached to the olfactometer containing treatments in place of the SPMEC (Figure 1).
- Deliver purified and humidified air through the SPMEC (or guillotine volatile chamber) via two pumps connected to an air delivery system (Figure 1). Maintain airflow rate at 0.1 L/min through both arms of the olfactometer.
- Employing olfactometer to test arthropod response to putative repellents. In the present description, a hemipteran insect, Asian citrus psyllid, Diaphorina citri, was used as a test arthropod. Response was tested to citrus volatiles alone or in combination with a repellent (dimethyl disulfide) and clean air as odor sources.
- Conduct all experiments at a standardized temperature and relative humidity.
- Prior to all tests, expose test subjects to clean air versus clean air or solvent versus clean air in the T-maze olfactometer to verify the absence of positional bias or an effect of solvent on behavior of the test subject.
- Assign an odor source randomly to one of the arms of the olfactometer at the beginning of each bioassay. Reverse this position after every 10 test subjects assayed, at minimum, to eliminate potential of positional bias.
- Test the response of a minimum of 30 (and up to 120) test subjects per treatment combination.
- Allow test subjects 100-300 sec to exhibit a behavioral response based on previously established response (latency) interval.
- Record if subject enters the treatment arm, control arm or remains in the release port or below the T-maze division.
- Score a treatment or control arm choice when a test subject crosses the division in the T-maze and moves into either olfactometer arm.
- Score a release arm choice when a test subject remains in the release port or below the T-maze division.
- Clean the olfactometer and connecting tubes thoroughly with 2% soap solution and bake the glass components at 200 °F between the use of different odor treatments.
- For assays in which putative repellent treatments are presented in the T-maze olfactometer with or without a chemical treatment versus clean air, compare the number of test subjects remaining at the release point and not entering the olfactometer between treatments by one way analysis of variance (ANOVA) followed by Tukey’s HSD test (α < 0.05). In cases in which the test subjects leave the release arm, compare the number choosing the control arm versus the treatment arm with Chi square (χ2) analysis at α < 0.05.
Representative Results
Attraction of Asian citrus psyllid (Diaphorina citri) adults to the odors of their natal host plant volatiles (citrus) is depicted in Figure 2A. Significantly (α < 0.05) more adults chose the arm of the T-maze olfactometer receiving odors from living citrus plants as compared with a blank (clean air) control.
An example of repulsion is shown in Figure 2B. In this case, the insects were exposed to one arm of the T-maze receiving citrus volatiles, while the second arm received volatiles from a citrus plant that was treated with the known repellent, dimethyl disulfide11. In this case, three types of behaviors were observed. The number of psyllids arrested at the release arm and not responding did not differ statistically from the number entering the arm receiving the odors from citrus plants alone (Figure 2B). However, no psyllids entered the arm containing the host plant volatile when co-presented with the repellent (Figure 2B).
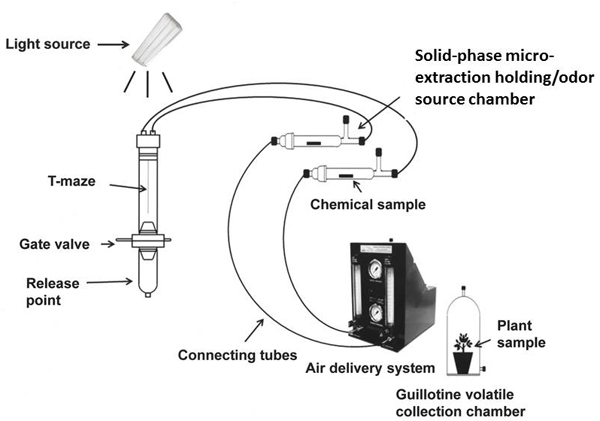
Figure 1. Schematic diagram of vertically oriented T-maze olfactometer joined to air delivery system and odor treatment release devices. The diagram is an adaptation of Figure 1 in Mann et al.10. A test subject is placed individually into the release chamber of the 2-port divided T-olfactometer. It moves towards the odors through a gate valve which allows controlled upward movement into the olfactometer and prevents the insect from crawling back into the release point area. The tube bifurcates 10 cm after the gate value. A positive response to an odor is recorded once the insect moves 0.5 cm into a specific side of the division. The guillotine volatile collection chamber may be used in place of a solid-phase micro-extraction holding/odor source chamber (SPMEC) as an odor source.
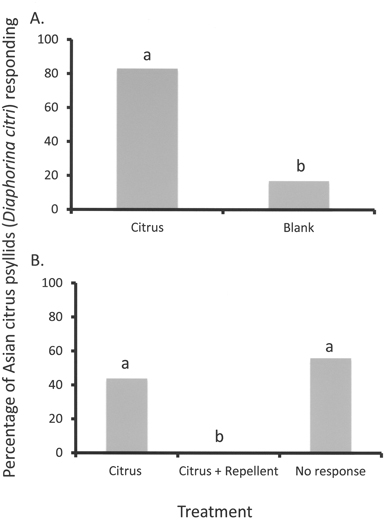
Figure 2. Response of Asian citrus psyllid (Diaphorina citri) adults to volatiles from citrus host plants versus clean air control (A) or citrus volatiles versus citrus volatiles co-released with a repellent (dimethyl disulfide) (B) in a vertically oriented T-maze olfactometer. Figure 2A: One-way analysis of variance (ANOVA) followed by Tukey’s HSD test (α < 0.05) was performed to compare the number of psyllid making a choice between either arm (2 treatments, n = 120). Columns indicated by different letters are significantly different from one another (α < 0.05). Figure 2B: One-way analysis of variance (ANOVA) followed by Tukey’s HSD test (α < 0.05) was performed to compare the number of psyllid making a choice between either arm or remaining in the release point (3 treatments, n = 120). Columns indicated by different letters are significantly different from one another (α < 0.05).
Discussion
Herein, an assay device and protocol are described for measuring the response of small arthropods (Insecta: Hemiptera: Psyllidae) to odorants. The method involves a choice test, allowing the insect to make a choice between two odorant fields in the case of assays evaluating a putative attractant. Furthermore, the test subject may display three types of behaviors by leaving the release arm and entering either one of two potential odor fields, or remaining in the release arm, in the case of repellency assays. The olfactometer allows for high throughput data collection because it takes advantage of the negative geotactic (tendency to move upward) and positive phototactic (tendency to move toward light) natural behavioral response of many arthropods. Although the present demonstration uses a psyllid insect test example, the assay can be readily adapted to arthropods broadly, both those that use flight or walking as predominant modes of transportation.
Olfactometers designed for measuring the response of insects were initially developed decades ago and have played a major role in elucidating insect attractants and repellents12. The specific designs of such olfactometers vary widely; however, variations on the general theme of the two-choice assay described here, as well as, similar Y-tube assays have been often used to measure arthropod response to chemicals. Larger flight tunnels that may cause sustained flight of insects9 have also resulted in collection of groundbreaking data elucidating the fundamental mechanisms of insect flight and orientation to semiochemicals, as well as, data informing practical use of pest control tools.
It is often necessary to tailor design an olfactometer and associated instrumentation for the biology of the specific arthropod test subject. Those olfactometers that can be used among a general group of insects are more useful than ones specific to a small group; however, sometimes the economic significance of a small group of insects dictates the need for development of a very specific olfactometer and assay technique. The currently described design builds on previously familiar arthropod techniques. It allows for a more standard two-choice assay than Y-tube bioassays in which the experimental arena or choice test takes the form of a Y-shaped glass device7. Typically, one arm of such a Y-tube will receive a treatment odorant, while the other will be left blank13. Variations on such olfactometers may include addition of multiple radiating arms14 and even addition of a soil medium for assaying the behavior of organisms moving through soil15. In developing such olfactometers and associated assays, it is important to consider how closely natural conditions are replicated and thus the true relevance of the assessed behavioral responses in terms of the test subjects’ biology. To a large degree, the data collected will only be as useful as the relevance of the behavioral bioassay with respect to the organism’s behavioral ecology16.
The currently described olfactometer and behavioral bioassay is specifically designed for a hemipteran insect that tends to initiate flight as short “jumps”16. The vertical orientation of the olfactometer and the light source arrangement facilitate initiation of insect movement and thus subsequent chemically-mediated orientation behavior can be assayed efficiently and with high throughput. This behavioral assay arrangement can likely be used for a broad array of flying or walking arthropod taxa or could be also easily modified to fit the need of non-arthropod organisms.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The Citrus Research and Development Foundation is acknowledged for providing funding. Thanks to Angel Hoyte and Michael Flores for conducting bioassays.
Materials
| Name of Equipment | Company | Catalog number | Comments |
| Two port divided T-olfactometer | Analytical Research Systems (ARS), Inc. Gainesville, FL | None(Custom made) | If unavailable from ARS, Southern Scientific (Gainesville, FL) also currently builds and distributes such equipment. |
| Solid-phase micro-extraction holding/odor source chamber with Teflon;-glass tube connectors. | Analytical Research Systems (ARS), Inc. Gainesville, FL | RV-R3 | See above comment |
| Air delivery system | Analytical Research Systems (ARS), Inc. Gainesville, FL | HADS-2AFM2C.4 | See above comment |
| Guillotine chamber | Analytical Research Systems (ARS), Inc. Gainesville, FL | L3GP3 | See above comment |
References
- Resh, R. H., Cardé, R. T. . Encyclopedia of Insects. , (2003).
- Aluja, M., Lesky, T. C., Vincent, C. . Biorational tree-fruit pest management. , (2009).
- Rodriquez-Saona, C., Stelinski, L. L., Peshin, R., Dhawan, A. K. Behavior-modifying strategies in IPM: Theory and Practice. Integrated Pest Management: Innovation – Development Process. , 261-311 (2009).
- Cardé, R. T., Millar, J. G. . Advances in insect chemical ecology. , (2004).
- Knight, A. L., Stelinski, L. L., Hebert, V., Gut, L., Light, D., Brunner, J. Novel dispensers simultaneously releasing pear ester and sex pheromone for disruption of codling moth (Lepidoptera: Tortricidae). Journal of Applied Entomology. 136 (1-2), 79-86 (2012).
- Mann, R. S., Tiwari, S., Smoot, J. M., Rouseff, R. L., Stelinski, L. L. Repellency and toxicity of plant-based essential oils and their constituents against Diaphorina citri Kuwayama (Hemiptera: Psyllidae). Journal of Applied Entomology. 136 (1-2), 87-96 (2012).
- Forbes, A. A., Powell, H. Q., Stelinski, L. L., Smith, J. J., Feder, J. L. Sequential sympatric speciation across trophic levels. Science. 323 (5915), 776-779 (2009).
- Kennedy, J. S. The visual responses of flying mosquitos. Proceedings of the Zoological Society of London A. 109 (4), 221-242 (1940).
- Miller, J. R., Roelofs, W. L. Sustained-flight tunnel for measuring insect responses to wind-borne sex pheromones. Journal of Chemical Ecology. 4 (2), 187-198 (1978).
- Mann, R. S., Rouseff, R. L., Smoot, J. M., Castle, W. S., Stelinski, L. L. Sulfur volatiles from Allium spp. affect Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), response to citrus volatiles. Bulletin of Entomological Research. 101 (1), 89-97 (2011).
- Onagbola, E. O., Rouseff, R. L., Smoot, J. M., Stelinski, L. L. Guava leaf volatiles and dimethyl disulfide inhibit response of Diaphorina citri Kuwayama to host plant volatiles. Journal of Applied Entomology. 135 (6), 404-414 (2011).
- Baker, T. C., Linn, C. E., Hummel, H., Miller, T. A. Wind tunnels in pheromone research. Techniques in Pheromone Research. , 75-110 (1984).
- Stelinski, L. L., Rodriguez-Saona, C., Meyer, W. L. Recognition of foreign oviposition marking pheromone in a multitrophic context. Naturwissenschaften. 96 (5), 585-592 (2009).
- Gökçe, A., Stelinski, L. L., Whalon, M. E. Behavioral and electrophysiological responses of leafroller moths to selected plant extracts. Environmental Entomology. 34 (6), 1426-1432 (2005).
- Ali, J. G., Alborn, H. T., et al. herbivore-induced plant volatile increases biological control activity of multiple beneficial nematode species in distinct habitats. PLoS ONE. 7 (6), e38146 (2012).
- Mann, R. S., Ali, J. G., et al. Induced release of a plant defense volatile ‘deceptively’ attracts insect vectors to plants infected with a bacterial pathogen. PLoS Pathogens. 8 (3), e1002610 (2012).

