Methods for Performing Crosses in Setaria viridis, a New Model System for the Grasses
Summary
We have developed a methodology for performing crosses in Setaria viridis (S. viridis). The method involves pruning the panicle prior to a hot water treatment to kill viable pollen. Crosses are performed following a well-controlled growth regime and typically result in the recovery of 1 to 7 cross-pollinated seed/s per panicle.
Abstract
Setaria viridis is an emerging model system for C4 grasses. It is closely related to the bioenergy feed stock switchgrass and the grain crop foxtail millet. Recently, the 510 Mb genome of foxtail millet, S. italica, has been sequenced 1,2 and a 25x coverage genome sequence of the weedy relative S. viridis is in progress. S. viridis has a number of characteristics that make it a potentially excellent model genetic system including a rapid generation time, small stature, simple growth requirements, prolific seed production 3 and developed systems for both transient and stable transformation 4. However, the genetics of S. viridis is largely unexplored, in part, due to the lack of detailed methods for performing crosses. To date, no standard protocol has been adopted that will permit rapid production of seeds from controlled crosses.
The protocol presented here is optimized for performing genetic crosses in S. viridis, accession A10.1. We have employed a simple heat treatment with warm water for emasculation after pruning the panicle to retain 20-30 florets and labeling of flowers to eliminate seeds resulting from newly developed flowers after emasculation. After testing a series of heat treatments at permissive temperatures and varying the duration of dipping, we have established an optimum temperature and time range of 48 °C for 3-6 min. By using this method, a minimum of 15 crosses can be performed by a single worker per day and an average of 3-5 outcross progeny per panicle can be recovered. Therefore, an average of 45-75 outcross progeny can be produced by one person in a single day. Broad implementation of this technique will facilitate the development of recombinant inbred line populations of S. viridis X S. viridis or S. viridis X S. italica, mapping mutations through bulk segregant analysis and creating higher order mutants for genetic analysis.
Introduction
S. viridis is an NADP-ME subtype C4 grass closely related to the bioenergy feed stock switchgrass (NAD-ME subtype), the grain crop foxtail millet, and the agricultural weed guinea grass 5. The 510 Mb genome of cultivated form of Setaria, S. italica, has recently been sequenced 1,2 and a 25x coverage genome sequence of the weedy relative, S. viridis, is in progress (unpublished). S. viridis has a number of characteristics that make it a potentially excellent model genetic system including a rapid generation time, small stature, simple growth requirements, and prolific seed production 3. Importantly, methods have recently been developed for both transient and stable transformation of S. viridis which provide the opportunity for creating transgenic plants 4. However, a major bottleneck in the development of genetic tools for S. viridis is its recalcitrance to outcrossing. To date, no standard protocol has been published that will permit rapid production of seeds from controlled crosses.
Genetic crosses in S. italica are usually performed by emasculation through removal of anthers from flowers 6,7,8 or through heat treatment of flowers of female parents 9-11. Following these treatments, anthers or panicles from non-treated flowers/male parents are gently abraded against stigmas releasing pollen. In emasculation, anthers are removed using fine forceps immediately after the floret opens and the anther is beginning to exsert, but has not yet shed pollen 6-8. Among the challenges of this latter method is the difficulty in performing emasculation in a narrow time interval between the opening and pollen shed of the flower. The duration of opening and closing of a single flower will vary depending on the accession and environmental condition, and can range from 7 min 6 to 2.5 hr 12 in S. italica. Since shedding of pollen occurs as anthers exsert from the floret, anthers need to be removed carefully and quickly, and therefore, it is hard to avoid contamination due to self-pollination. In addition, as pollinations are performed immediately after emasculation, the number of crosses that can be performed per person/per day is limited.
As an alternative method, mature panicles can be dipped in warm water to heat-kill developing pollen grains 9,10,13,14 with the advantage of performing crosses on a large number of panicles. However, the temperature and duration of heat treatment varies widely from different experiments (e.g. 47 °C for 10 min 14 and 42 °C for 20 min 10). Furthermore, no systematic analyses on the efficacy of heat-mediated emasculations have been published. Thus, a standard and simple method for performing genetic crosses in S. viridis would greatly accelerate genetic resource development and establishment of S. viridis as a model system within the research community.
We report the development and optimization of a standard protocol for performing genetic crosses in S. viridis. Plants are grown under controlled environments to synchronize flower development and increase reproducibility of the procedure. Crosses are performed using a transgenic S. viridis line as the male parent containing the GUS reporter gene 4 to facilitate the identification of successful controlled genetic crosses. The GUS transgene is driven by a rice Ubiquitin promoter which enables the scoring of F1 seeds immediately after harvesting and thus provides an easy qualitative assay to determine the efficiency of emasculation and pollination. We have employed a simple heat treatment with warm water for emasculation accompanied by labeling of flowers that have been emasculated to eliminate seeds resulting from newly developed flowers after emasculation. After testing a series of heat treatments at permissive temperatures and varying the duration of dipping in warm water, we have established an optimal temperature and time durations of 48 °C from 3-6 min. A pre-dawn cold treatment was found to promote synchronous anthesis in both parents in order to facilitate cross pollination. We have also discussed the key steps in the protocol and the future application of this method to other Setaria accessions.
Protocol
1. Growth of S. viridis
- Set the growth chamber conditions to 31 °C/22 °C (day/night), 12 hr light/12 hr dark, 50% relative humidity with a pre-dawn treatment of 15 °C from 8:30 AM to 9:00 AM. (Figure 1). Under these conditions, S. viridis A10.1 takes approximately 21-22 days from seed sowing to anthesis.
- Fill flats (4 x 9 cells) with Metro Mix 360 and remove one cell for watering.
- Lightly mist water on soil surface.
- Sow seeds on the surface of soil, one seed per cell.
- Cover the seeds with a layer of soil (about 0.5 cm)
- Mist the surface of soil and water flats from the bottom. Keep watering from the bottom after seed sowing.
- For each cross that will be performed at least three plants will be used as female parents and nine plants will be used as male parents. Seed sowing should be staggered as all plants will not flower on the same day. Seed sowing is recommended each day over a three-day period.
- Water the plants 1-2 times a day, which is dependent on the size of the plants and soil condition. Excess water in soil is not favorable for optimum growth of the plants, and may lead to fungal growth in soil and damage of leaf tissue.
- Fertilize the plants twice a week, using Jack’s 15-16-17 at a concentration of 100 ppm.
2. Trimming and Labeling of Panicle Before Emasculation – Day 1
- In the afternoon or evening prior to pollination, move plants from the growth chamber to the lab (Temperature (T): 24.06 °C± 0.13 °C, relative humidity (RH) 21.79% ± 1.15%).
- Select the primary panicles having 1 flower to 1/3 of flowers on the panicle that have already bloomed.
- In this study the four domains within the panicle are defined as follows (Figure 2A; tip, distal middle, proximal middle and base).
- Ideally, choose a panicle with 1-5 flowers that have bloomed, cut off the tip of the panicle (distal end), and remove all the flowers in the proximal middle and base sections by using spring scissors or fine forceps.
- If a panicle has about 1/10 to 1/5 of flowers that have bloomed, cut off the tip and trim flowers from the base of the panicle, only retain the lower section of distal middle and upper section of proximal middle for further trimming.
- If a panicle has about 1/4 to 1/2 of flowers that have bloomed, cut off the distal middle section and only retain the proximal middle section for further trimming.
- Use surgical scissors to lightly trim the bristles.
- Remove all flowers that have bloomed and all immature flowers leaving approximately 20-30 flowers/panicle that are evenly distributed in the region (Figure 3). Practice care to retain the bristles in the region that will be pollinated in order to protect florets from possible heat damage. For each spikelet, retain the upper most mature floret. The upper most floret will have a higher probability of anthesis at the time of pollination (Figure 2B).
- Mark one side of each flower with a red permanent marker and record the number of florets on the panicle (Figure 2B).
- Flag the panicle with the following information: the date of emasculation, the duration and the temperature at which the emasculation is carried out.
- Once the panicle is trimmed, remove all tillers of each plant to ensure reallocation of more resources to the development of the main panicle.
3. Emasculation with Warm Water-dipping – Day 1
- Set the water bath to 48 °C ± 0.1 °C for the heat treatment.
- Gently bend the trimmed panicles and dip them in warm water at 48 °C for 3-6 min. Use caution not to break the stem of the panicle.
- About 3-4 panicles can be dipped together at one time, while maintaining sufficient space between the panicles for uniform distribution of heat. It is essential to submerge the entire panicle in warm water during heat treatment. The flag leaf (the leaf subtending the panicle that provides the nutrients to the panicle) should not come into contact with warm water to prevent heat damage to leaf tissue.
- Once the emasculation is performed for the desired duration, remove panicles from the water bath and gently shake off the water from the panicle.
- Place a customized micro-perforated bread bag to fully cover the emasculated panicle and secure it with a twist-tie. Micro-perforated bread bags can be customized according to the size of the panicle (include in video).
- Place plants back in the growth chamber until the following day.
4. Crossing/pollination after Emasculation – Day 2 and Day 3
- At 9:00 am on day 2 and day 3, move female (emasculated) and male parents from the growth chamber to the lab (T: 24.06 °C ± 0.13 °C, RH: 21.79% ± 1.15%) to perform crosses.
- Observe and add another red dot to flowers that have bloomed or are blooming using a red permanent marker. Record the total number of bloomed flowers per panicle.
- Record the date of pollination and the name of the male parent on the tag to keep track of what crosses are performed.
- Observe the peak time of anthesis in male parents. Under our chamber conditions (Figure 1) flowers on the panicles of the male parent begin synchronous opening around 10:10 AM. From the time of opening of flowers, the complete exsertion of anthers and release of pollen will occur after 20 min.
- The ideal stage for pollination is the stage at which the pollen of the male parent is released. The flowers will close after 20 min from pollen release. Hence, the duration for anthesis is about 40 min from the time of opening of flowers. The color of pollen changes from yellowish white to brown over a period of about 30 min after the flower closes.
- Start pollination immediately once yellowish white anthers are visible on flowers of the male parent. Pollinations are performed on day 2 and day 3 by using one of the three techniques:
- Panicle-to-panicle pollination: using one detached panicle as the male, gently rub the panicle together with the emasculated panicle to facilitate pollination and discard the male panicle to avoid contamination. Repeat the pollination process until each emasculated panicle has been pollinated by 2-3 panicles.
- Anther-to-stigma pollination: using forceps pick a flower that is blooming with yellowish white anthers or pick anthers and physically touch the stigma of the flower on the female parent. Use one flower from the male parent to pollinate one flower on the emasculated panicle. Repeat the pollination process until each stigma on the emasculated panicle has been pollinated by a few (about 2-3) flowers/anthers. Wearing a magnifying visor will render better visibility to perform the pollination.
- Between the pollinations of different male parents, dip the forceps in 95% ethanol followed by wiping with Kimwipes to avoid contamination due to carry-over of pollen between different crosses.
- Panicle binding: After emasculation, select 3-4 panicles on the male parent that will bloom the following day. Bind them together with the emasculated panicle of the female parent using a twist-tie. Position the emasculated panicle slightly below the panicle of the male parent. Place a glassine bag over the panicles and secure the bag at the base with a paper clip. This may be completed on day 2 once the panicles of the male parent start anthesis.
- During the blooming time in the morning of day 2 and day 3, flick or shake the glassine bag to facilitate pollination. Continue to flick or shake panicles every 15 min throughout the entire duration of anthesis in the morning (between 9:00 AM-11:00 AM). Remove panicles of the male parent after pollination and label the opposite side of the bloomed flowers on the emasculated panicle. Record the number of bloomed flowers on the panicle of each female parent.
- Once the pollination has been performed, place a customized micro-perforated bread bag to cover the panicle of the female parent and secure at the base by using a twist-tie after pollination until seed harvesting.
5. Seed Harvesting
- Harvest seeds after 2 weeks (14-16 days) from the day of pollination. Place the tag in the same bag of the seeds. Seeds that have red markings on both sides represent the seeds that resulted from the controlled genetic cross. These are expected to be outcross progeny.
- Discard any seeds without red markings as they represent the seeds that result from newly developed flowers after emasculation. Seeds with red markings only on one side will likely represent the seeds resulting from self-pollination as they were not blooming at the time when the controlled crosses were performed.
- Dry seeds at 30 °C for 2 days in a seed dryer. Store dried seeds in the lab (T: 24.06 °C ± 0.13 °C, RH: 21.79% ± 1.15%) or in a seed chamber (T: 4.0 °C ± 1.0 °C, RH: 20% ± 1%).
Representative Results
In this study, we used the sequenced S. viridis accession A10.1 as the female parent, and a transgenic S. viridis line (also A10.1) containing the GUS reporter gene 4 as the male parent for all pollinations. An optimized growth regime was used to synchronize flowering as shown in Figure 1. We have tested several combinations of temperature and time of heat treatment for emasculation and observed that about 40-80% of the flowers retained on the emasculated panicle bloomed on the 2nd day while the remainder bloomed on the 3rd day (Figure 2). However, if the heat treatment was too severe, for an example, 49 °C for 4 min, very few or no flowers bloomed on the 2nd day. Cross-pollination was performed on the 2nd or 3rd day after emasculation, which was dependent on the peak time of anthesis of male parents.
It was observed that young flowers continued to develop after emasculation, matured and self-pollinated 3-6 days after controlled pollinations. After 7-10 days post-pollination, flowers that are white in color are visible on the panicle. These white flowers are unfertilized due to either heat damage or poor pollination. At the same time, the flowers that have been successfully pollinated begin to bear dark seeds and start shattering after 14 days. The dark seeds with red markings harvested on the 14th day after pollination could be clearly distinguished from the seeds without red markings that were still green and did not shatter. If harvesting is delayed, the anthecium of the seeds with and without red markings both turn dark, which makes it difficult to quickly distinguish the seeds with red markings in harvested pools.
To optimize the crossing protocol several heat treatment experiments were performed as summarized in Table 1. An average of 0-10 seeds/panicle were recovered from various heat treatment experiments. After harvesting, seeds were dried at 30 °C for 2 days in a seed dryer followed by the removal of the anthecium using an anthecium remover. After the anthecium was removed, putative F1 seeds were stained with GUS staining solution, and outcross progeny stained blue color in 1-2 hr (Figure 2F). Based on these results, we recommend a heat treatment of 48 °C for 3-6 min. Furthermore, we recommend that crosses be performed on both day 2 and day 3 following emasculation.
To examine the effectiveness of this technique in performing crosses with other Setaria accessions or species, crosses between S. viridis A10.1 and eight diverse S. viridis accessions and one S. pumila accession were performed. Although days to flowering varied among the accessions we found that flowers of all eight S. viridis accessions and one S. pumila accession begin opening between 10:00-11:00 AM with a peak opening time at 10:20-11:00 AM after the predawn cold treatment. This indicates that the predawn cold treatment can be applied to diverse Setaria accessions. A heat treatment of 48 °C for 5-6 min was used to emasculate S. viridis A10.1 which was used as the female parent for all crosses performed . We recovered from one to 12 seeds from crosses between A10.1 and three of the diverse S. viridis accessions and six seeds from a single cross between S. viridis A10.1 and a single S. pumila accession. However, at this time we have not determined if these seed resulted from a successful testcross or were the result of self-contamination. Regardless, these preliminary results suggest that the growth conditions used for S. viridis A10.1 can be used to generate crosses with diverse S. viridis accessions and possibly other species of Setaria.
| Temp heat treat (°C) | Time heat treat (min) | # crosses | Avg. # of flowers after trim | Day of pollination (day after emasc) | Avg. # seeds (red)/ panicle (mean) | Avg. % Gus (+)/ seeds (red) | Avg. # hyb seeds (Gus+) | Min # hyb seeds | Max# hyb seeds |
| 47 | 10 | 2 | 27 | 2nd day | 0 | 0 | 0 | 0 | 0 |
| 48 | 3 | 3 | 36 | 2nd day | 5 | 60 | 3 | 1 | 5 |
| 4 | 3 | 25 | 3rd day | 10 | 50 | 5 | 3 | 7 | |
| 5 | 8 | 25 | 2nd day | 5 | 60 | 3 | 1 | 6 | |
| 6 | 3 | 25 | 2nd day | 4 | 75 | 3 | 0 | 4 | |
| 7 | 4 | 24 | 2nd day | 3 | 33 | 1 | 0 | 4 | |
| 7 | 1 | 25 | 3rd day | 4 | 75 | 3 | N/A | N/A | |
| 49 | 1 | 3 | 23 | 2nd day | 5 | 25 | 1 | 0 | 2 |
| 2 | 7 | 26 | 2nd day | 8 | 25 | 2 | 0 | 4 | |
| 3 | 5 | 22 | 2nd day | 0 | 0 | 0 | 0 | 1 | |
| 4 | 4 | 25 | 2nd day | 0 | 0 | 0 | 0 | 0 |
Table 1. Optimization of heat treatment for emasculation. Results of altering both temperature and time of heat treatment. Pollinations were performed one (2nd day) or two days (3rd day) following emasculation and successful outcrosses scored with GUS staining
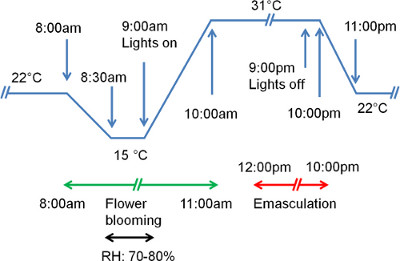
Figure 1. Optimized growth chamber conditions with a pre-dawn cold treatment. Temperature and time periods for optimal growth and synchrony of flower production are shown. Green and red arrows show optimal window for performing crosses and emasculation, respectively.
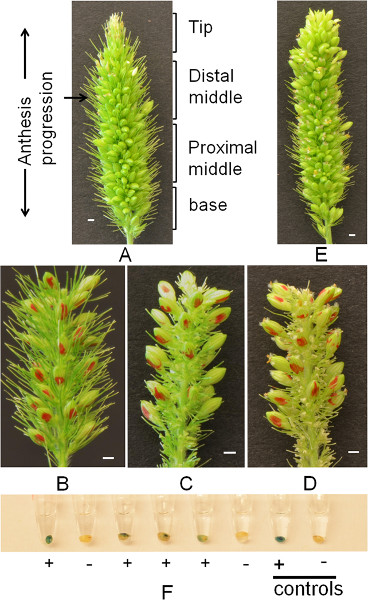
Figure 2. Panicle preparation for crossing and GUS staining of outcross progeny. (A) A panicle of Setaria viridis A10.1 before trimming, showing approximately 1/10 of flowers opened. The four sections and directionality in progression of anthesis along the panicle is shown. (B) A trimmed panicle prior to emasculation with bristles retained. (C) An emasculated panicle after removal of bristles. (D) An emasculated panicle on day 2 post-emasculation, showing flowers that are blooming (picture taken at 10:30 AM). (E) A panicle of the male parent (transgenic Setaria viridis A10.1 line carrying a β-glucuronidase (GUS) reporter gene) that is blooming after pre-dawn treatment (picture taken at 10:30 AM). A through E, scale bars = 1 mm. (F) Putative outcross progeny after GUS staining for 2 hr followed by distaining in 70% (v/v) ethanol. The two seeds from the right are negative and positive controls, respectively.
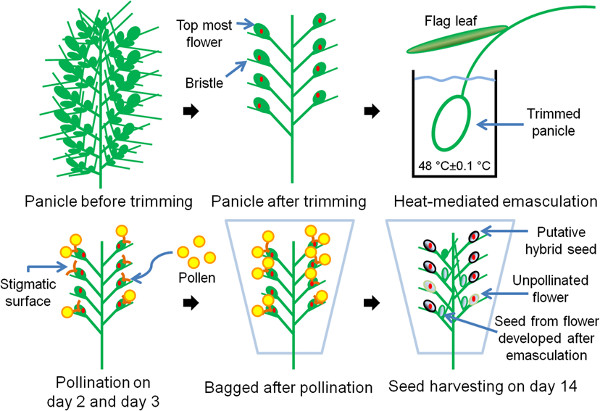
Figure 3. Flow chart of the protocol A schematic representation of the major steps involved in performing S. viridis crosses.
Discussion
Importance of pre-dawn treatment
To obtain a high frequency of outcross progeny it is essential to have highly synchronous anthesis of male and female parents. Initially, we cycled temperature and light regimes (31 °C/22 °C, day/night) and used the S. viridis accession A10.1 for all pollinations. Under these conditions, most flowers open early in the morning before the temperature rises to 31 °C, though a few flowers open randomly between 8:00 AM-8:00 PM. Previous studies in S. italica indicate that time of day, location and season contribute to variation in anthesis 7,15,16. Siles et al.6 noted that anthesis was associated with rapid changes in temperature and humidity, but not with low temperature and high humidity, per se, as concluded in previous studies 7,15. Therefore, we employed a pre-dawn cold treatment of 15 °C for 30 min (from 8:30 AM-9:00 AM) to mimic the natural pre-dawn condition. This pre-dawn cold treatment aided in synchronizing anthesis in the parents. We observed that despite setting the chamber for relative humidity levels of 50%, the relative humidity inside the chamber increased to a level above 70% as the temperature dropped from 22 °C to 15 °C and continued to stay above 70% until the temperature gradually rose to 31 °C after 9:30 AM. At 9:00 AM, emasculated female parents and plants of male parent are brought to the lab (T: 24.06 °C ± 0.13 °C, RH: 21.79% ± 1.15%). Flowers of the male parents start opening around 10:10 AM, and pollination can be performed at 10:30 AM-11:00 AM. A detailed list of all reagents and equipment is provided in Table 2.
Importance of panicle preparation for emasculation
Under the chamber conditions in this study (Figure 1), the duration of primary panicle blooming ranged from 2-3 days. Typically, flowers located on the distal middle of the inflorescence (Figure 2A) open first and opening precedes proximally and distally. The ideal number of flowers to be retained on the panicle is 20-30. Caution should be taken during the removal of immature flowers so that only well-developed flowers (the topmost flower or biggest on the spikelet 17) are retained. Labeling with red marker on both sides of the flowers helps to efficiently distinguish putative outcross progeny from seeds from newly developed flowers after emasculation. In addition, it is important to leave the bristles on the panicles before emasculation to protect the florets from extensive heat damage; however, as an option, they can be removed after emasculation using spring scissors in order to facilitate pollination, especially when a panicle binding technique is employed (Figures 2C and 2D).
Optimized temperature and treatment duration for warm water treatment
The basis for emasculation through warm water dipping is that pollen is more sensitive to heat than the stigmatic surface. However, the duration and temperature of heat treatments may vary among Setaria species and accessions. In general, a higher temperature with a shorter treatment time or a lower temperature with a longer treatment time will have the same effect on emasculation. It has been reported that a heat treatment of 42 °C for 20 min 10 or 47 °C for 10 min 14 rendered S. italica pollen non-viable, but the efficiency of these treatments was not determined. We have developed our protocol following the hypothesis that under an optimized temperature and duration of treatment, pollen of all flowers retained on the female parent will be non-viable while stigmas will remain receptive. However, after comparing and analyzing the effects of several temperatures and treatment periods (Table 1), we found that the sensitivity to the heat treatment varies among the flowers retained on each panicle, thus it is hard to completely eliminate seeds resulting from self-pollination. We conclude that the efficiency of producing outcross progeny is the greatest when treatments are performed at 48 °C for 3-6 min in S. viridis accession A10.1.
Peak time of anthesis and cross-pollination
When the flowers reach maturity and are about to open, anthers are yellowish white in color and pollen is shed as soon as anthers exsert from the flowers 8. Anthers gradually turn brown after pollen is shed as the flowers begin to close. Once released, the viability of pollen is unknown. Therefore, it is critical to use the pollen from opening or freshly opened flowers on the male parent as soon as possible. Under our chamber conditions (Figure 1), the majority of flowers of the male parents start to open at 10:10 AM and the opened flowers shed pollen at 10:30 AM. Thus, the desirable window for performing pollination is between 10:30 AM and 11:00 AM. The stigmas remain outside the glumes after flowers close and may be receptive to pollen. This has been previously observed and confirmed in S. italica that the stigmas are receptive for about 48 hr post-flower opening by Siles et al.6, so it is important to bag panicles following heat treatment and after performing controlled crosses. As discussed above, we recommend performing pollinations on both Day 2 and Day 3 post-emasculation.
Methods for pollination
We compared the efficiency of three pollination techniques. If blooming flowers are not limiting, panicle-to-panicle pollination is the most efficient technique and will yield a higher number of outcross progeny. If blooming panicles are limiting, a higher frequency of outcross progeny will be produced when the anther-to-stigma method is used. We have obtained outcross progeny from both methods successfully. The “binding panicles” method has been used in crossing S. italica 6 but we found this method to be the least efficient method as the time window for pollen shedding of the male parent is short. Moreover, there is less control over pollination and the bristles on S. viridis panicles may also hinder the movement of pollen onto the stigmatic surface.
Seed harvesting, drying and storage
After harvesting, seeds should be dried at 30-33 °C for 2 days. Anthecium should be removed for GUS staining, if necessary. Seeds should be stored in a dry, cool location (T: 24.06 °C ± 0.13 °C, RH: 21.79% ± 1.15%) for short-term storage (less than two years) or in a seed chamber (T: 4.0-10 °C ± 1.0 °C, RH: 20% ± 1%) for long-term storage. Poor storage conditions may result in low viability rates 8. We have observed that the germination rate of S. viridis A10.1 is approximately 5% when sown 4 days after harvesting, but can be increased to 90-96% after storage in the laboratory (T: 24.06 °C ± 0.13 °C, RH: 21.79% ± 1.15%) for 110 days post-harvesting followed by a three-day stratification at -80 °C to break seed dormancy. For the stratification at -80 °C, dry seeds can be placed in an airtight container (e.g. micro-centrifuge tube or coin envelope in a sealed plastic bag) and kept at -80 °C for 3 days before planting. After 16 months of storage in the laboratory (T: 24.06 °C ± 0.13 °C, RH: 21.79% ± 1.15%), germination percentages of 90-95% can still be obtained. In addition, to break the dormancy, seeds can be dried at 30-33 °C for 2 days after harvesting, and then given three-day stratification at -80 °C followed by the removal of anthecium before planting. After these treatments, seed germination rates of up to 33% can be achieved.
Advantages, limitations and possible modifications
Here we provide the first standard protocol for performing crosses in S. viridis A10.1 by using a heat treatment for emasculation. In contrast to physical emasculation, this protocol is less invasive and relatively easy to establish in a lab. It usually takes about 15 min to trim one panicle and about 15 panicles can be trimmed and crossed/person/day. Assuming an average of 3-5 outcross progeny/panicle can be recovered under optimized conditions, a total of 45-75 outcross progeny can be produced by one person in a single day. Furthermore, this technique can be applied to crosses in other Setaria species, though additional optimizations will be likely. If growth chamber space is limited, plants can be grown in green house or growth chambers without a pre-dawn treatment until the panicle emerges before they are moved to the optimized chamber conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Sankalpi Warnasooriya and Amy Humboldt for critical reading and editing of the manuscript. This work was supported by grants from Department of Energy (DE-SC0008769) and the National Science Foundation (IOS-1127017).
Materials
| Name | Company | Cat. Number | |
| Scissors, spring | World Precision Instruments, Inc | 14126 | |
| Forceps, Dumostar Biology Polished | SPI Supplies | TD5BP-XD | |
| Surgical Scissors | F.S.T (Fine Science Tools) | 14005-12 | |
| Europack Clear Polypropylene Micro-Perforated Crusty Bread Bags 6″x28″ | http://www.pjpmarketplace.com | 361001 | |
| Flats | T.O. Plastics | 715401C | |
| metro mix 360 | Hommert International | 10-0356-1 | |
| Jack’s 15-16-17 | Hommert International | 07-5925-1 | |
| Kimwipes | VWR International | 34120 | |
| Sharpie Ultra Fine Point Permanent Markers, Red | Staples | 37002 | |
| Donegan DA-10 OptiVisor Headband Magnifier, 3.5x Magnification, 4″ Focal Length | Amazon | DA-10, B0015IP380 | |
| 12″24/7 Packaging Hand Impulse Sealer Heat Seal Machine Poly Sealing Free Element Grip | Amazon | N/A | |
| water bath | VWR scientific | Model: 1166 | |
| BDW walk in plant growth chamber | Conviron | BDW 40 |
References
- Bennetzen, J. L., et al. Reference genome sequence of the model plant Setaria. Nat Biotechnol. 30, 555-561 (2012).
- Zhang, G., et al. Genome sequence of foxtail millet (Setaria italica) provides insights into grass evolution and biofuel potential. Nat Biotechnol. 30, 549-554 (2012).
- Li, P., Brutnell, T. P. Setaria viridis and Setaria italica, model genetic systems for the Panicoid grasses. J Exp Bot. 62, 3031-3037 (2011).
- Brutnell, T. P., et al. Setaria viridis: a model for C4 photosynthesis. Plant Cell. 22, 2537-2544 (2010).
- Doust, A. N., Kellogg, E. A., Devos, K. M., Bennetzen, J. L. Foxtail millet: a sequence-driven grass model system. Plant Physiol. 149, 137-141 (2009).
- Siles, M. M., Baltensperger, D. D., Nelson, L. A. Technique for artificial hybridization of foxtail millet [Setaria italica (L.) Beauv.]. Crop Sci. 41, 1408-1412 (2001).
- Li, H. W., Meng, C. J., Liu, T. N. Problems in the Breeding of Millet (Setaria Italica (L.) Beauv.). Agron. J. 27, 963-970 (1935).
- Willweber-Kishimoto, E. Interspecific relationships in the genus setaria. Contributions from the Biological Laboratory, Kyoto University. 14, 1-41 (1962).
- Chang, L. P. Studies on flowering and hybridization technique in Setaria. Nungyeh-hsueh Pao (Jour. Agric.) Act. Agric. Sin. 9, 68-76 (1958).
- Darmency, H., Pernes, J. Use of wild Setaria viridis (L.) Beauv. to improve triazine resistance in cultivated S. italica (L.) by hybridization. Weed Research. 25, 175-179 (1985).
- Sakai, S., Shin, C. Artificial hybridization of Setaria italica by hot water treatment. Bull. Fac. Agric. Kagoshima Univ. , 28-37 (1955).
- Malm, N. R., Rachie, K. O. . Setaria millets: A review of the world literature S.B. , 513-529 (1971).
- Miyaji, Y., Samura, T. The influence of atmospheric humidity on flowering and pollination in Setaria italica. Bull. Fac. Agric. Kagoshima Univ. , 1-6 (1954).
- Wang, Z. M., Devos, K. M., Liu, C. J., Wang, R. Q., Gale, M. D. Construction of RFLP-based maps of foxtail millet, Setaria italica (L.). P. Beauv. Theoret. Appl. Genetics. 96, 31-36 (1998).
- RangaswamiAyyangar, G. N., Narayanan, T. R., Seshadri Sarma, P. Studies in Setaria italica (Beauv.), the Italian millet. Part I. Indian J. Agr. Sci. , 561-571 (1933).
- Heh, C. M., Mei, T. F., Yang, S. S. Anthesis of Millet, Setaria Italica (L.) Beauv. Agron. J. 29, 845-853 (1937).
- Doust, A. N., Devos, K. M., Gadberry, M. D., Gale, M. D., Kellogg, E. A. The genetic basis for inflorescence variation between foxtail and green millet (poaceae). Genetics. 169, 1659-1672 (2005).

