Isolation of Cortical Microglia with Preserved Immunophenotype and Functionality From Murine Neonates
Summary
One key to successful investigation of microglial biology is the preservation of microglial immunofunction ex vivo during isolation from CNS tissue. Isolating microglia via rotary shaking results in highly pure and immunofunctional cell cultures as assessed by fluorescent imaging, immunocytochemistry, and ELISA following microglia activation with the proinflammatory stimuli lipopolysaccharide (LPS) and Pam3CSK4 (Pam).
Abstract
Isolation of microglia from CNS tissue is a powerful investigative tool used to study microglial biology ex vivo. The present method details a procedure for isolation of microglia from neonatal murine cortices by mechanical agitation with a rotary shaker. This microglia isolation method yields highly pure cortical microglia that exhibit morphological and functional characteristics indicative of quiescent microglia in normal, nonpathological conditions in vivo. This procedure also preserves the microglial immunophenotype and biochemical functionality as demonstrated by the induction of morphological changes, nuclear translocation of the p65 subunit of NF-κB (p65), and secretion of the hallmark proinflammatory cytokine, tumor necrosis factor-α (TNF-α), upon lipopolysaccharide (LPS) and Pam3CSK4 (Pam) challenges. Therefore, the present isolation procedure preserves the immunophenotype of both quiescent and activated microglia, providing an experimental method of investigating microglia biology in ex vivo conditions.
Introduction
Microglial cells, the surveillance macrophages of the CNS parenchyma, comprise approximately 12% of the total cell population of the adult mammalian brain. Microglia are derived from yolk sac myeloid precursor cells and vary in cell density and morphology in different cytoarchitectural regions within the adult CNS1-5. In a healthy adult brain, microglia are small, ramified or polar cells with fine, dynamic processes. In contrast to peripheral macrophage morphology, microglia demonstrate a quiescent, low-profile phenotype in healthy brains that may appear as cellular inactivity; however, in vivo imaging studies demonstrate that microglial processes dynamically extend and retract to monitor their microenvironment in a manner reminiscent of "sampling and surveying"6,7.
Microglia are highly and differentially responsive to environmental and pathophysiological alterations in the brain, switching from a surveillant to an effector state—commonly regarded as their resting and activated states, respectively. This switch in activation can be mediated by engagement of membrane-bound pattern recognition receptors (PRRs), such as toll-like receptors (TLRs), which respond to pathogen-associated molecular patterns (PAMPs), namely bacterial- and viral-derived lipoproteins, nucleic acids, and carbohydrates8-11. In addition to PAMPs, PRRs have also been shown to induce microglial activation against sterile, nonpathogenic molecules known as danger/damage-associated molecular patterns (DAMPs), which represent a perturbation in CNS homeostasis, such as cellular damage12-16. Once engaged, PRRs initiate an intracellular signaling cascade that results in changes in microglial cell morphology and gene expression; specifically, activated microglia adapt an amoeboid-like phenotype, translocate the p65 NF-κB subunit (p65) to the cell nucleus, and upregulate the production and secretion of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β), along with reactive oxygen species (ROS)16-24. Although integral in the innate immune response of the CNS, these secreted molecules have also been found to increase neuronal oxidative stress, thereby inducing and exacerbating neurodegeneration in diseased states such as Parkinson's and Alzheimer's disease25-29.
However, the mechanisms of microglial activation in pathological states are not completely understood. Therefore, isolation of microglia is a powerful investigative tool into these biological processes, as many in vivo features of microglial activation can be recapitulated in culture. Several methods are available for isolating microglia, including isolation via a Percoll gradient following enzymatic digestion of CNS tissue30,31. However, enzymatic digestion can alter the immunophenotype of the cells by reducing cell-surface antigen expression32, and results in lower cell yield per animal than the method described herein. Specifically, we report an average microglia yield per pup cortex of 7.5 x 105 cells, while previously reported isolation methods from whole CNS via a Percoll gradient yield 3-5 x 105 cells30,33,34. The present procedure circumvents the use of digestion enzymes by isolating microglia based on their low-adherence properties, thereby preserving the microglial immunophenotype and functionality.
In the present study, we describe the isolation of microglial cells from mixed glial cultures derived from neonatal heterozygous CX3CR1-GFP (CX3CR1-GFP+/-), and C57BL/6 murine cortices via mechanical agitation on a rotary shaker, an extension of previously published method24,35. We utilize the former mouse strain for easy visualization of microglia, as these mice express GFP under the control of the endogenous Cx3Cr1 locus - a monocyte-specific promoter36-38. This method produces highly pure microglial cultures with a preserved immunophenotype ex vivo as demonstrated by morphological changes, nuclear translocation of p65, and secretion of TNF-α when challenged with bacterial lipopolysaccharide (LPS) or Pam3CSK4, TLR4 and TLR1/2 agonists, respectively.
Protocol
Prior to starting this protocol, collect neonatal mice at postnatal days 1-3 (P1-3) in a sterile container nested with original cage bedding for protection and warmth. It is important to work quickly and efficiently through this protocol to optimize microglial yield. Please see Table 1 for complete reagent list.
1. Preparation of instruments, Culture Media, and Dishes
- Prepare 10 ml/pup Microglia Complete Media (MCM; 1x Minimal Essential Medium Earle's [MEM] supplemented with: 1 mM L-glutamine, 1 mM sodium pyruvate, 0.6% v/v D-(+)-glucose, 100 μg/ml Penicillin/Streptomycin (P/S), 4% v/v Fetal Bovine Serum (FBS), 6% v/v Horse Serum), and add to each T-75 flask (1 flask/pup); cap and place horizontally into an incubator (37 °C, 5% CO2) to equilibrate for 1 hr. It is essential to equilibrate this media in flask(s) before starting the dissection.
- Prepare, aliquot, and warm in 37 °C water bath MCM for dissection (6 ml/pup) and in a separate conical tube MCM for cell resuspension (2 ml/pup). Prepare, aliquot, and chill on ice Mincing Media (MM; HBSS supplemented with: 100 μg/ml P/S, 0.01 M HEPES; 3 ml/pup), and Dissecting Media (DM; MEM supplemented with 100 μg/ml P/S; 2 ml/pup).
- Sterilize the following surgical tools by submersion in 70% ethanol for 10 min: scissor #1 (1; to decapitate), forcep #1 (2; to secure nose and remove cortices), scissor #2 (1; nonangled; to open scalp), forcep #2 (1; to remove scalp), forcep #3 (1; to remove skull), forcep #4 (2; to remove meninges), scissor #3 (1; to mince brain tissue). Allow to air dry on a sterile pad.
- Wipe down the horizontal-airflow workstation and dissection microscope with 70% ethanol.
- Dispense DM into 2 sterile, 100 mm Petri dishes for capturing head (3 ml; 1 dish/3 pups) and dissection (1 ml; 1 dish/pup; chilled on ice prior to dissection). Dispense MM into 1 sterile, 100 mm Petri dish for mincing brain tissue (1 ml; 1 dish/pup).
2. Brain Tissue Dissection
- Prior to euthanasia, gently sanitize neck and head of pup with 70% ethanol using a lab tissue. With scissor #1, quickly euthanize pup by removing the head; capture head in previously prepared Petri dish. Euthanize and complete the dissection of one pup before proceeding to the next animal.
- Transfer the head to the chilled dissection dish; under dissection microscope secure the head by pinching the nose using forcep #1 (alternatively, the head can be secured by piercing the forceps through the eye socket); open scalp using scissor #2 by creating a Y-cut: begin from base of head and at an angle toward the eyes. Use forcep #3 to gently remove scalp by peeling laterally, exposing the skull.
- Using forcep #3, gently remove connective tissue at base of head. When base of skull is exposed, carefully open skull by threading one arm of forcep in between the brain and skull (following the interhemispheric fissure); grip; gently pull the skull upwards to tear open.
- Disrupt connective tissue under ventral side of brain using forcep #1, and subsequently remove brain using the same tool. Lift the brain out of skull by using an upward force under the ventral side of the brain.
- Keep the brain in its anatomical position: dorsal side facing upwards to visualize the cortices under the dissection microscope; secure the brain in place by inserting tips of forceps in the intersection of the midbrain and the cortex; completely remove cortical meninges with forcep #4, then proceed to remove the ventral meninges. Once the meninges have been completely removed, separate the cortices from the remainder of the brain using forcep #4. It is essential to completely remove meningeal tissue to maximize microglia purity and yield.
3. Brain Tissue Mincing
- Transfer cortices to previously prepared mincing dish; continue to mincing step using sterile technique in Class II Biological Safety Cabinet.
- Mince brain tissue using scissor #3; transfer minced tissue to a 15 ml conical tube; rinse scissors and mincing dish with 1 ml of MM each, collect and dispense rinses into the 15 ml conical tube containing the minced tissue. This rinse step will maximize the yield of microglia by ensuring that all residual tissue is transferred to conical tube. Leave tissue suspension undisturbed for 1-2 min, allowing minced tissue to settle at bottom of tube. Do not exceed 2 min as MM does not contain essential nutrients.
- Carefully remove and discard 2 ml of supernatant with generic 1,000 μl micropipettor with a standard pipette tip, making certain not to disrupt the settled minced-tissue.
- Add 3 ml of MCM to the minced-tissue; triturate the tissue with full force using a generic 1,000 μl micropipettor with a standard pipette tip. No discernable solid tissue should remain when trituration is complete. Add another 3 ml of MCM with the same pipette tip, collecting residual triturated sample from the pipette tip.
4. Growing Cortical Cells
- Centrifuge the minced tissue to pellet cells (215 x g; 5 min; RT; clinical centrifuge with swinging bucket rotor). Return pelleted cells to the biological safety cabinet, remove and discard the supernatant; gently resuspend the cell pellet (2 ml/tube; MCM); add the cell resuspension to the previously prepared T-75 flask; cap the flask and place it horizontally into a humidified, standard tissue culture incubator (37 °C, 5% CO2). Allow the cells to incubate for 24 hr.
- After 24 hr, gently tap flask against palm of hand to disrupt tissue debris. Check cells before and after tapping to ensure complete removal of debris. Set flask vertical; remove and discard media; add fresh MCM to the bottom of the flask (12 ml; 37 °C); cap and place back into the incubator, horizontally.
- Check cells daily for contamination and to monitor growth.
- At approximately 17-21 days in vitro (DIV), microglial cells will be ready for harvest from the mixed glial culture. To determine if cells are ready, examine cells under an inverted phase-contrast microscope. Microglia are ready for harvest when at ~40% confluency. They appear as small, bright, spherical cells, growing as a monolayer on top of other glia.
5. Isolation of Microglia from Mixed Glial Culture
- To isolate microglia, vigorously tap flask against laboratory bench. This agitation helps separate microglia from other glial cells due to the low-adherence properties of microglia.
- Seal T-75 flask caps with Parafilm and rotate the mixed glial cultures (200 rpm; 37 °C; 5 hr) using a temperature-controlled, nonhumidified rotary shaker. Do not allow cells to shake for longer than 5 hr. Visually inspect that microglia have lifted off the flask surface after 5 hr, using an inverted phase-contrast microscope. Microglia are bright, spherical, free-floating cells. There will be a residual layer of astrocytes and oligodendrocytes adhering to the bottom of the flask.
- Collect supernatant media containing microglia into a sterile conical centrifuge tube; pellet microglia (215 x g; 5 min; swinging bucket rotor).
- Resuspend the microglial pellet in warm Microglial Growth Medium (MGM; MEM supplemented with: 1 mM sodium pyruvate, 0.6% v/vglucose, 1 mM L-glutamine, 100 μg/ml P/S, 5% v/v FBS; ~2 ml/flask), determine the cell concentration using a hemocytometer, and plate 1 x 105 cells/well in a 24-well plate format on plastic or sterile glass cover slips (500 μl/well; MGM). Using this procedure the average microglia yield is 7.5 x 105 cells/pup cortex.
- 24-hr post-plating, remove all of the media and refeed cells with fresh MGM (37 °C) to remove floating cells and debris. This step is critical to cell survival and to maintain quiescent microglia. Cells may be utilized for experimentation immediately after refeeding.
6. Example Treatments
- Expose cells to 10 ng/ml of Pam3CSK4 (Pam), 10 ng/ml lipopolysaccharide (LPS) or vehicle control; incubate for 2 or 24 hr in humidified incubator (37 °C, 5% CO2).
7. Analyzing Microglia for Functionality Ex vivo via Immunofluorescent Imaging and Immunocytochemistry
- After treatment, harvest cell culture media in microfuge tubes (1.5 ml) and centrifuge using a tabletop microcentrifuge (2 min; 900 x g) to clear media. Transfer the supernatant to a clean microfuge tube; assay immediately, or store at -20 °C until ready to use. Analyze TNF-α concentration in cell culture supernatant via commercially available mouse TNF-α ELISA kit.
- Wash cells with phosphate buffered saline (PBS), and fix cells with 4% (w/v) paraformaldehyde/4% (w/v) sucrose/PBS pH 7.4 (4% PFA; 15 min; RT). Following fixation, wash cells with PBS for 5 min with gentle shaking on an orbital shaker.
- Immunofluorescence/Immunocytochemistry. After fixation and PBS wash, process microglia for immunocytochemistry. All steps carried out with gentle shaking. Permeabilize cells in PBS/0.1% triton X-100; block cells in PBS/10% normal goat serum for one hour. Incubate cells O/N at 4 °C with rabbit anti-NF-κB antibody (p65 subunit; 1/1,250) or rabbit anti-Iba-1 antibody (1/750) in blocking buffer. Visualize antigen:antibody complex following incubation with fluorescently conjugated goat anti-rabbit IgG secondary antibody (1 hr; 1/1,000). Remove unbound secondary antibody by washing with PBS/0.1% triton X-100 (3x 5 min).
- Counter stain cells with 4',6-Diamidino-2-Phenylindole/PBS (DAPI; 0.1 μg/ml) to visualize the cell nucleus. All microglia derived from CX3CR1-GFP+/- mice express GFP. Utilize a 350 nm wavelength filter to visualize DAPI; a 488 nm wavelength filter to visualize GFP; and a 594 nm wavelength filter to visualize p65 and Iba-1 immunocomplexes.
- Mount cover slips using aqueous mounting solution and visualize microglia using fluorescent microscopy.
Representative Results
Retaining the immunophenotype and functionality of microglia ex vivo during isolation is critical to be able to utilize these cells as an investigatory model for microglial biology. In order to demonstrate the successful preservation of microglia immunofunctionality using the present method, we isolated cortical microglia from P3 neonates (CX3CR1-GFP+/-and C57BL/6) and treated cultures with either LPS or Pam.
As illustrated in Figure 1A, isolation of microglia via agitation through rotary shaking preserves the quiescent phenotype attributed to microglia in normal in vivo conditions. To assess microglial purity, CX3CR1-GFP+/- cell cultures were stained for the macrophage antigen Iba-1 and counter stained with DAPI in order to visualize the cell nucleus. As shown in Figures 1A and 1B, under low magnification, microglia isolation via the presently described method results in a highly pure cell culture, as greater than 95% of cells exhibit colocalization of GFP, Iba-1, and DAPI.
We next sought to confirm the responsiveness of microglia ex vivo by challenging cells with LPS. In comparison to vehicle treatment, LPS-treated CX3CR1-GFP+/- microglia adapted a stark morphological change from a small, bipolar phenotype to an amoeboid-like shape, as shown in Figure 1B. This morphological change is also recapitulated in cortical microglia isolated from C57BL/6 neonates treated with Pam (Figure 2). This conserved morphological response between genetically distinct microglia, activated with different stimuli, underscores the reproducibility and applicability of the present protocol in regards to various experimental models.
Even though this change in morphology is indicative of activation39, it is necessary to determine if microglia isolated by rotary shaking retain their ability to translocate p65 upon activation, indicating the preservation of functional TLR-mediated intracellular signaling. To confirm this signaling cascade, we treated microglia isolated from C57BL/6 neonates with Pam. As illustrated in Figure 2, immunocytochemical staining for p65 evidenced that, under normal conditions, p65 is diffusely localized throughout the cytoplasm, whereas after a 2 hr stimulation with Pam, p65 immunoreactivity dramatically shifted localization to the cell nucleus.
Having established that microglia isolated via the method presented herein preserve the molecular mechanisms to translocate p65 to the nucleus, we next wanted to confirm that isolated microglia retain their biochemical immunofunctionality by testing their ability to secrete a hallmark proinflammatory cytokine, TNF-α, upon activation. As demonstrated in Figure 3, in comparison to vehicle-treated microglia, cells exposed to Pam or LPS increase the secretion of TNF-α into the culture media (P<0.0001; one-way ANOVA), indicative of functional TLR1/2 andTLR4 signaling pathways, respectively.
Therefore taken together, these data establish that isolation of microglia via rotary shaking results in highly pure cell cultures with preserved immunophenotype and functionality, ex vivo.
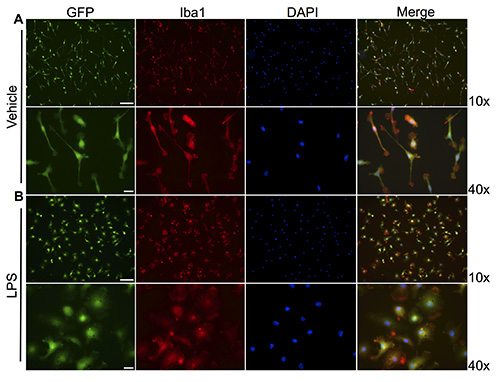
Figure 1. Rotary shaking yields high purity microglial cultures with preserved immunophenotype. Microglial cells were isolated from CX3CR1-GFP+/- neonatal mice at P3 and treated with either vehicle (A), or bacterial LPS (B) for 24 hr. Cells were fixed with 4% PFA, immunocytochemically stained for Iba-1, and counter stained with DAPI. (A) Microglia treated with PBS exhibit a bipolar, quiescent phenotype. (B) Upon LPS challenge, microglia adapt an amoeboid-like phenotype, indicative of activation. Merged channel in panels (A) and (B) under 10X magnification demonstrate that >95% of cells are GFP/Iba-1 positive cells. 10X scale bar: 100 μm; 40X scale bar: 20 μm. Click here to view larger image.
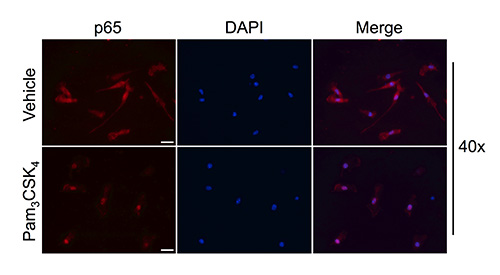
Figure 2. Microglia isolated via rotary shaking translocate NF-κB to cell nucleus upon Pam challenge. Microglial cells were isolated from C57BL/6 neonates at P3 and treated with vehicle or Pam for 2 hr. Cells were fixed with 4% PFA and stained for p65 via immunocytochemistry. Cells were counter stained with DAPI to visualize the cell nucleus. In vehicle treated microglia, p65 is localized throughout the cytoplasm, whereas stimulation with Pam induces the nuclear translocation of p65. 40X scale bar: 20 μm. Click here to view larger image.
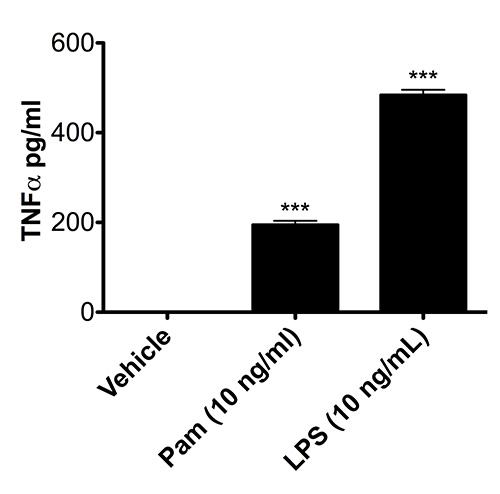
Figure 3. Microglia isolated with rotary shaking release TNF-α upon exposure to Pam or LPS. Microglial cells were isolated from C57BL/6 neonatal mice at P3 and treated with vehicle, Pam, or LPS for 2 hr. Cell culture supernatants were collected and analyzed via ELISA for TNF-α release. *** P< 0.0001 (n=2; one-way ANOVA). Data are presented as means ± SD.
Discussion
The present procedure offers an effective method for the isolation of cortical microglia from neonatal mice. This procedure has a two-fold benefit of 1) preserving microglia immunophenotype and functionality, as determined by fluorescent imaging, immunocytochemistry, and ELISA; and, 2) allowing microglia to mature in the presence of other glial cells (astrocytes and oligodentrocytes) prior to isolation, which may facilitate important cell-cell interactions during the glial culture maturation period. Importantly, isolated cells exhibited high viability and uniformity with preserved functionality and immunophenotype ex vivo.
There are several steps that need to be performed with particular attention and diligence for successful isolation of microglia with the highest purity and yield. These steps are: 1) maintaining a sterile, healthy culture throughout the entire experimentation procedure; 2) removing all meninges from the cortices during microdissection; 3) proper washing of mincing plate and scissors after mincing cortices; 4) equilibrating MCM in T-75 flasks in incubator before beginning microdissection; 5) refeeding 24 hr post-isolation.
Since microglia are the resident immune cells of the CNS, they are highly responsive to environmental insults. Therefore, avoiding culture contamination with pathogenic agents is critical to preserving the immunophenotypic and functional attributes of quiescent microglial cells. Daily inspection of cells to monitor contamination and growth is integral to avoiding compromised cell cultures and to gain a better understanding of microglial growth-rate. Furthermore, it is necessary to refresh MGM 24-hr post plating of microglia in order to maintain cell viability and functionality.
A major obstacle to overcome in isolating microglia is to ensure that the minced CNS tissue is free of endothelial cells, which, if present, can impede microglial proliferation by forming dense colonies in the glial cultures. For this reason, it is critical to thoroughly remove the CNS meninges during microdissection of the brain. In our experience, the meninges of P3 neonates are removed with greater ease than P1 neonates, as the tissue is more developed and easier to visualize under the dissection microscope. Additionally, it is important to avoid wetting the dorsal side of the brain with DM after removing the brain from the skull. This can be done by keeping the brain in anatomical position (dorsal side up) when transferring it to DM. Keeping the dorsal side of the brain dry allows for better visualization and easier removal of the meninges.
We have found that the most effective way to remove the meninges is to insert the force #4 in the most posterior portion of the interhemispheric fissure, and peel meninges off, laterally. Once the dorsal meninges have been cleared, we then remove the ventral meninges by inverting the brain (ventral-side up), and gently grasping the olfactory bulbs and peeling back towards the posterior portion of the brain. We then remove any remaining ventral meningeal tissue by gently peeling and tweezing in a lateral motion. Subsequent separation of the cortex from the rest of the brain is crucial for isolating only cortical microglia. The procedure detailed herein is also flexible in that it can be used to isolate microglia from subcortical regions, as well. Though, it is important to note that cell yield may be lower due to the variability of microglia density indifferent cytoarchitectural brain regions40.
We have found that to maximize the final yield of microglia it is important to rinse and collect the rinse from the materials used in the mincing process. These rinses ensure the complete collection of all residual tissue that may have remained on the mincing scissors, Petri dish, and the pipette tip used to aliquot the minced tissue into the conical tube. It is important that these rinses take place immediately after transferring the minced CNS tissue to the conical tube, to optimize cell viability. It is also critical to cell viability that the cells do not stay in MM for longer than 2 min since MM is devoid of essential nutrients.
Lastly, it is crucial to prepare the T-75 flasks with MCM and place them in the humidified incubator (37 °C; 5% CO2) prior to starting the microdissection protocol. TheT-75 flask has a porous lining under its cap that allows for the exchange of gases, facilitating media equilibration before the addition of mixed glial cultures. We have found that failure to do this results in a suboptimal microglial harvest. It is also important to seal the T-75 flask cap with Parafilm prior to placing the flask on the rotary shaker in order to minimize gaseous exchange in and out of the flask while rotating.
The present procedure details an effective method of isolating cortical microglia from neonatal mice resulting in a higher average yield of microglia (7.5 x 105 cells/pup cortex) than previously reported methods utilizing digestive enzymes and/or a Percoll gradient (3-5 x 105 cells/whole CNS)30,33,34 . As evidenced by fluorescent imaging, immunocytochemistry, and ELISA, the microglia isolated via this procedure are capable of undergoing morphological and biochemical responses when exposed to immunogenic stimuli such as Pam and LPS. Therefore, the procedure described herein provides an experimental method of investigating microglial biology in ex vivo conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by NIEHS R01ES014470 (KMZ).
Materials
| Glucose | Sigma | G8270 | Make 20% Stock Solution with MilliQ water; filter sterilize; store at 4 °C; shelf life: 3-6 months. Used to make MCM and MGM. |
| Sodium pyruvate 100 mM (100x) | Hyclone | SH30239.01 | Store at 4 °C. Used to make MCM and MGM. |
| Penicillin/Streptomycin 10,000 units/ml (100x) | Gibco | 15140-122 | Store at -20 °C. Used to make MCM, MGM, MM, and DM. |
| L-glutamine 200 mM (100x) | Gibco | 25030-081 | Store at -20 °C. Used to make MCM and MGM. |
| Fetal Bovine Serum (Defined) | Hyclone | SH30070.03 | Filter sterilize; store at -20 °C. Used to make MCM and MGM. |
| Minimum Essential Medium Earle's (MEM) | Cellgro | 15-010-CV | Without L-glutamine. Contains Earle's salts. Used to make MCM, MGM, and DM. |
| Horse Serum | Gibco | 16050 | Filter sterilize; store at -20 °C. Used to make MCM and MGM. |
| Hanks' Balanced Salt Solution (HBSS) | Cellgro | 21-021-CV | Without calcium and magnesium. Store at 4 °C. Used to make MM. |
| HEPES 1 M | Gibco | 15630-031 | Store at 4 °C. Used to make MM. |
| T-75 Flask | Corning | 430641 | |
| 4',6-Diamidino-2-Phenylindole, dilactate (DAPI) | Invitrogen | D3571 | Used to stain cell nucleus. |
| Rabbit anti-Iba1 | Wako | 019-19741 | Used at 1/750 dilution for ICC staining of Iba1. |
| Rabbit anti-NFκB (p65) | Abcam | 7970 | Used at 1/1250 dilution for ICC staining for p65. |
| Alexafluor 594 Goat anti-Rabbit IgG (H+L) | Invitrogen | A11012 | Used at 1/1000 dilution for visualization of antigen:antibody complex in ICC. |
| 10 ml Disposable Serological Pipet | Fisher Scientific | 13-678-11E | |
| 50 ml Disposable Centrifuge Tube | Fisher Scientific | 05-539-8 | |
| 15 ml Disposible Centrifuge Tube | Fisher Scientific | 05-539-12 | |
| Sterile Polystyrene Petri Dish | Fisher Scientific | 875713 | 100 mm x 15 mm |
| Scissor: Straight Metzembaum (scissor #1) | Roboz Surgical | RS-6010 | 1; 5 inch; used for removing head |
| Scissor: Vannas (scissor #2) |
Fine Science Tools | 15000-08 | 1; non-angled; 2.5mm cutting edge; used to open scalp |
| Scissor: Student Vannas (scissor #3) | Fine Science Tools | 91501-09 | 1; curved; used to mince brain tissue |
| Forcep: Dumont #7 (forcep #1) |
Fine Science Tools | 91197-00 | 2; used to secure nose and remove cortices |
| Forcep: Dumont #2 (forcep #2) |
Fine Science Tools | 11223-20 | 1; used to remove scalp |
| Forcep: Dumont #3 (forcep #3) |
Fine Science Tools | 11231-30 | 1; used to remove skull |
| Forcep: Dumont #5a (forcep #4) |
Fine Science Tools | 11253-21 | 1; used to remove meninges |
| Table of specific equipment | |||
| Name of Equipment | Name of Company | Catalogue Number | Comments |
| Zoom Stereo Dissection Microscope | Olympus | SZ4060 | Microscope is placed inside Laminar-Horizontal Flow Cabinet |
| Laminar-Horizontal Flow Cabinet | Nuaire | NU-201-330 | |
| Biological Safety Cabinet | Labconco | 3440001 | Class II |
| Water-Jacketed CO2 Incubator | VWR | 97025-836 | Set to 37 °C, 5% CO2 |
| Swing-out buckets | Fisher Scientific | 75006441 | To be used with Swing-out rotor |
| Swing-out Rotor | Fisher Scientific | 75006445 | Max Radius: 19.2 (cm) |
| Sorvall Legend RT+ Centrifuge (clinical centrifuge) |
Fisher Scientific | 75-004-377 | With swing-out rotor |
| AccuSpin Micro 17 microcentrifuge (tabletop microcentrifuge) |
Fisher Scientific | S98645 | With microliter rotor (24 x 1.5/2.0 ml; Cat #: 75003524) |
References
- Ranshoff, R. M. P. V. H. Microglia Physiology: Unique Stimuli, Specialized Responses. Annu. Rev. Immunol. 27, 119-145 (2009).
- Lawson, L. J., Perry, V. H., Dri, P., Gordon, S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 39, 151-170 (1990).
- Gomez Perdiguero, E., Schulz, C., Geissmann, F. Development and homeostasis of "resident" myeloid cells: The case of the microglia. Glia. 61, 112-120 (2013).
- Kierdorf, K., et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273-280 (2013).
- Saijo, K., Glass, C. K. Microglial cell origin and phenotypes in health and disease. Nature reviews. Immunology. , 11-775 (2011).
- Davalos, D., et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752-758 (2005).
- Nimmerjahn, A., Kirchhoff, F., Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 308, 1314-1318 (2005).
- Hu, S., et al. Cytokine and free radical production by porcine microglia. Clin. Immunol. Immunopathol. 78, 93-96 (1996).
- Muzio, M., Polentarutti, N., Bosisio, D., Prahladan, M. K., Mantovani, A. Toll-like receptors: a growing family of immune receptors that are differentially expressed and regulated by different leukocytes. J. Leukocyte Biol. 67, 450-456 (2000).
- Lee, S. J., Lee, S. Toll-like receptors and inflammation in the CNS. Curr. Drug Targets. Inflamm. Allergy. 1, 181-191 (2002).
- Block, M. L., Zecca, L., Hong, J. S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57-69 (2007).
- Halle, A., et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857-865 (2008).
- Chen, G. Y., Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826-837 (2010).
- Duewell, P., et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464, 1357-1361 (2010).
- Stewart, C. R., et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4. 11, 155-161 (2010).
- Beraud, D., et al. alpha-Synuclein Alters Toll-Like Receptor Expression. Front. Neurosci. 5, 80 (2011).
- Banati, R. B., Gehrmann, J., Schubert, P., Kreutzberg, G. W. Cytotoxicity of microglia.. Glia. 7, 111-118 (1993).
- Combs, C. K., Karlo, J. C., Kao, S. C., Landreth, G. E. beta-Amyloid stimulation of microglia and monocytes results in TNF alpha-dependent expression of induciblenitric oxide synthase and neuronal apoptosis. J. Neurosci. 21, 1179-1188 (2001).
- Kim, Y. S., Joh, T. H. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 38, 333-347 (2006).
- Colton, C., Wilcock, D. M. Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets. 9, 174-191 (2010).
- Nakajima, K., Tohyama, Y., Kohsaka, S., Kurihara, T. Ceramide activates microglia to enhance the production/secretion of brain-derived neurotrophic factor (BDNF) without induction of deleterious factors in vitro. J. Neurochem. 80, 697-705 (2002).
- Uesugi, M., Nakajima, K., Tohyama, Y., Kohsaka, S., Kurihara, T. Nonparticipation of nuclear factor kappa B (NFkappaB) in the signaling cascade of c-JunN-terminal kinase (JNK)- and p38 mitogen-activated protein kinase (p38MAPK)-dependent tumor necrosis factor alpha (TNFalpha) induction in lipopolysaccharide (LPS)-stimulated microglia. Brain Res. , 1073-1074 (2006).
- Beraud, D., et al. Microglial Activation and Antioxidant Responses Induced by the Parkinson’s Disease Protein alpha-Synuclein. J. Neuroimmun. Pharmacol. 8, 94-117 (2013).
- Su, X., et al. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging. 29, 1690-1701 (2008).
- Minghetti, L., Levi, G. Microglia as effector cells in brain damage and repair: focus on prostanoids and nitric oxide. Prog. Neurobiol. 54, 99-125 (1998).
- Hirsch, E. C. Glial cells and Parkinson’s disease. J. Neurol.. 247 (2), 58-62 (2000).
- Liu, B., et al. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann. NY Acad. Sci. 962, 318-331 (2002).
- Shie, F. S., Nivison, M., Hsu, P. C., Montine, T. J. Modulation of microglialinnate immunity in Alzheimer’s disease by activation of peroxisome proliferator-activated receptor gamma. Curr. Med. Chem. 16, 643-651 (2009).
- Rogers, J., Mastroeni, D., Leonard, B., Joyce, J., Grover, A. Neuroinflammation in Alzheimer’s disease and Parkinson’s disease: are microglia pathogenic in either disorder. Int. Rev. Neurobiol. 82, 235-246 (2007).
- Cardona, A. E., Huang, D., Sasse, M. E., Ransohoff, R. M. Isolation of murinemicroglial cells for RNA analysis or flow cytometry. Nat. Protoc. 1, 1947-1951 (2006).
- Ford, A. L., Goodsall, A. L., Hickey, W. F., Sedgwick, J. D. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigenpresentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 154, 4309-4321 (1995).
- Ford, A. L., Foulcher, E., Goodsall, A. L., Sedgwick, J. D. Tissue digestion with dispase substantially reduces lymphocyte and macrophage cell-surface antigen expression. J. Immunol. Methods. 194, 71-75 (1996).
- Pino, P. A., Cardona, A. E. Isolation of brain and spinal cord mononuclear cells using percoll gradients. J. Vis. Exp. , (2011).
- Veremeyko, T., Starossom, S. C., Weiner, H. L., Ponomarev, E. D. Detection of microRNAs in microglia by real-time PCR in normal CNS and during neuroinflammation. J. Vis. Exp. , (2012).
- Su, X., Federoff, H. J., Maguire-Zeiss, K. A. Mutant alpha-synuclein overexpression mediates early proinflammatory activity. Neurotox. Res. 16, 238-254 (2009).
- Jung, S., et al. Analysis of fractalkine receptorCX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106-4114 (2000).
- Imai, T., et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 91, 521-530 (1997).
- Cardona, A. E., et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917-924 (2006).
- Streit, W. J., Walter, S. A., Pennell, N. A. Reactive microgliosis. Prog. Neurobiol. 57, 563-581 (1999).
- Frank, M. G., Wieseler-Frank, J. L., Watkins, L. R., Maier, S. F. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus:immunophenotypic and functional characteristics. J. Neurosci. Methods. 151, 121-130 (2006).

