A Proboscis Extension Response Protocol for Investigating Behavioral Plasticity in Insects: Application to Basic, Biomedical, and Agricultural Research
Summary
The Proboscis Extension Response (PER) conditioning protocol, developed for the honey bee (Apis mellifera), provides an ecologically-relevant and easily quantifiable means for studying several different mechanisms of learning in many insect species.
Abstract
Insects modify their responses to stimuli through experience of associating those stimuli with events important for survival (e.g., food, mates, threats). There are several behavioral mechanisms through which an insect learns salient associations and relates them to these events. It is important to understand this behavioral plasticity for programs aimed toward assisting insects that are beneficial for agriculture. This understanding can also be used for discovering solutions to biomedical and agricultural problems created by insects that act as disease vectors and pests. The Proboscis Extension Response (PER) conditioning protocol was developed for honey bees (Apis mellifera) over 50 years ago to study how they perceive and learn about floral odors, which signal the nectar and pollen resources a colony needs for survival. The PER procedure provides a robust and easy-to-employ framework for studying several different ecologically relevant mechanisms of behavioral plasticity. It is easily adaptable for use with several other insect species and other behavioral reflexes. These protocols can be readily employed in conjunction with various means for monitoring neural activity in the CNS via electrophysiology or bioimaging, or for manipulating targeted neuromodulatory pathways. It is a robust assay for rapidly detecting sub-lethal effects on behavior caused by environmental stressors, toxins or pesticides.
We show how the PER protocol is straightforward to implement using two procedures. One is suitable as a laboratory exercise for students or for quick assays of the effect of an experimental treatment. The other provides more thorough control of variables, which is important for studies of behavioral conditioning. We show how several measures for the behavioral response ranging from binary yes/no to more continuous variable like latency and duration of proboscis extension can be used to test hypotheses. And, we discuss some pitfalls that researchers commonly encounter when they use the procedure for the first time.
Introduction
Many insects learn about ecologically relevant stimuli, and they then change their behavioral responses to those stimuli in order to adapt to new predictive relationships in their environment. Several different mechanisms can underlie this behavioral plasticity (e.g., nonassociative, associative/Pavlovian, and operant/instrumental1). These types of plasticity differ in how the stimuli or behaviors are associated with important events, such as the occurrence of food, a mate, or danger. Understanding these forms of plasticity is very important for basic research into how the nervous system changes to encode new memories2. It is also important for understanding the adaptive behaviors of insects that are important vectors of disease (e.g., tsetse and mosquito) and insects that are agriculturally important, either in crop production (honey bees) or as pests.
Studying behavioral plasticity in any animal requires a level of experimental control over a number of variables that is not achievable in the field1. It requires the development of a robust conditioning protocol that can be employed under more controlled conditions, yet which are still relevant to behavior under natural conditions. The honey bee (Apis mellifera) is an excellent model for how to develop a protocol for performing controlled analyses of behavioral plasticity3,4. The Proboscis Extension Response (PER) in honey bees is a natural behavioral reflex in which the honey bee extends its proboscis in response to antennal stimulation with a sugar solution. During normal foraging behavior, PER occurs when the honey bee finds nectar in a flower. Fortunately, honey bees will readily exhibit this simple and easily quantifiable behavior in the laboratory. This makes it possible to study, in a controlled setting, the mechanisms that influence this ecologically relevant behavior5. PER can also be used within a conditioning protocol to investigate stimulus perception and learning and memory under different treatment conditions, which are designed to reveal the behavioral and neural mechanisms that underlie the plasticity6.
Since the first studies by Kuwabara7, PER conditioning has been widely used to reveal nonassociative, associative and operant mechanisms that underlie behavioral plasticity in honey bees8. These mechanisms are identical to those revealed in studies of freely flying honey bees9. Unlike studies of freely flying honey bees, PER conditioning protocols can be coupled with electrophysiology10,11 or live-cell fluorescence imaging12-14 of the brain. Furthermore, PER protocols allow for experimental manipulation of neural pathways via pharmacological or molecular genetic treatments to test hypotheses about the roles of specific components of the network, such as neuromodulators15,16. PER protocols have also provided an important way to evaluate the sublethal effects of environmental conditions as well as toxins on health and foraging efficiency of honey bees17.
This procedure describes two odor delivery methods in parallel. Method 1 is a version of the odor and unconditioned stimulus (sucrose) delivery that provides an inexpensive and technically simple method for presenting the odor and sucrose reward. This method is good for basic training and when automation is not possible. It is an excellent way to introduce this technique to a classroom or teaching laboratory. During conditioning for experiments involving more difficult tasks and coupled physiological assessments of odor perception, learning, or memory, it is very important to accurately and precisely regulate the onset, duration delivery timing of stimuli. For the most reliable stimulus delivery, use a means of automating the odor delivery and a precise method of reward delivery. Method 2 employs automated odor delivery and more precise sucrose delivery. It is technically more sophisticated and requires more for the initial setup than Method 1, but it significantly increases the consistency of the timing and quantity of stimuli used for conditioning and should be used whenever possible.
Protocol
1. Odor (Conditioned Stimulus) Cartridge Setup
- At the beginning of the experiment, setup multiple odor cartridges for all the different odors needed for the conditioning trials.
- Use a fume hood for odor dilution and odor cartridge preparation. Never open bottles of odors outside of that fume hood, since the odors will spread rapidly throughout the lab and potentially expose the honey bees to the odor before conditioning. Also, wear gloves while setting up the odor cartridges and wash thoroughly afterward to avoid exposing the honey bees to odors while handling them.
- Make sure each odor cartridge is clearly labeled with a color-coded label of the odor for which it is first used. Never use an odor cartridge (syringe barrel or plunger) for more than one odor, since there may be residual odor on the cartridge.
- Preparing Odors and Odor Mixtures
- Dilute odors in either hexane or mineral oil to the desired concentration.
NOTE: Altering the concentration of the odor or presenting mixtures of two or more odorants can allow for investigation of additional aspects of how bees sense odors and learn the conditioned association. Odor mixtures can be as simple as a 50:50 binary mixture or as complex as several odors that mimic natural olfactory stimuli. Reducing the concentration of the odor also increases the difficulty of the task.- Odors will become depleted with reuse of the cartridge (Figure 2). To avoid problems from depletion make enough cartridges to be able to switch to a new cartridge every 10-12 uses.
- Dilute odors in either hexane or mineral oil to the desired concentration.
- Preparing Odor Cartridges
- Method 1
- For this method, use 20 ml plastic syringes with a 15 mm diameter circle of filter paper pinned to the rubber end of the plunger for odor cartridges.
- Remove the plunger from the syringe and use a pushpin to attach the piece of filter paper to the end of the plunger.
- Use a micropipette to place 10 μl of the odor onto the filter paper, and reinsert the plunger into the syringe barrel. Push the plunger to the 15 ml mark on the syringe barrel.
- Method 2
- For this method, use 1 cc glass or plastic tuberculin syringes (or modified pipettes of similar volume and shape) with a strip of filter paper inside and a rubber or silicone restrictor in the wide end of syringe barrel for the odor cartridges.
- Remove the plunger from a 1 cc tuberculin syringe barrel. The syringe barrel will be the body of the odor cartridge.
- Remove the black rubber tip from the syringe plunger and cut off the closed end of the rubber tip.
NOTE: This rubber ring will act as a restrictor to reduce suction from the airflow in the arena, which will prematurely draw odor out of the cartridge and over the restrained honey bee. Alternately, silicone tubing (4.8 mm outer diameter) cut into 5 mm sections work as restrictors.
- Rinse the inside of the syringe barrel and the rubber/silicone restrictor with 70% ethanol (to remove much of the residual odor from previous use) and let them air dry.
- Place a 0.2 x 4 cm strip of filter paper into the wide opening of the barrel so the paper extends 1-2 cm beyond the opening.
- Use a micropipette to place 3-10 μl of the odor onto the filter paper without letting the odor touch the end of the syringe barrel. The pipette tip may be reused for the same the odor, but make sure to use a fresh tip to set up the next odor.
- Turn the wide opening of the syringe barrel upward so the filter paper slides rapidly into the tube. Insert the restrictor into the wide opening of the syringe barrel.
- Make sure each odor cartridge is clearly labeled with a color-coded label of the odor it is first used for. Never use an odor cartridge (syringe barrel or restrictor) for more than one odor, since there may be residual odor on it.
- Ball up the end of a lint-free tissue, soak it with 70% ethanol, and use it to thoroughly wipe the outer surface of the cartridge to remove any residual odor on the outside of the cartridge.
- Method 1
2. Collecting, Restraining, and Feeding the Bees
- Preparing Bee Restraining Harnesses
- Make simple restraining harnesses for honey bees out of plastic soda straws, hard plastic tubing, or machined metal tubing (all are approximately 0.9 cm inner diameter and have 1-2 mm wall thickness). Use sturdier harnesses for procedures involving surgery.
- Cut 3 cm sections of the tubing. Then, partially trim away approximately half of the upper 1 cm of the harness to make it easier to hold the bee in place while fastening the harness.
- Cut a 0.2 x 6 cm strip of duct tape and attach one end to one side of the bee harness, leaving the other end free.
- Collecting Worker Bees
- Collect workers from the entrance to the colony as they pause before departing from the colony or as they return from foraging.
NOTE: Placing a piece of wire mesh, with holes just large enough for worker bees to crawl through, over the entrance slows the returning workers down and makes it easier to capture them before they enter the colony. - Place a scintillation vial over the bee until it flies up into it. Hold the vial with its opening horizontally or facing down while the lid is fastened so the bee doesn’t fly up out of the vial. Place only one bee in each vial. Make sure the lid has a hole in it to allow for adequate gas exchange.
- Collect the bees quickly to minimize the time they spend in the vials. Keep the vials with bees in them in a small box in the shade.
- Transfer the box with the vials and bees to the lab, and place the vials into an ice-water slurry until the bees stop moving. Once a bee is immobile, remove the vial from the ice immediately to avoid overexposure to cold, and place the bee into a restraining harness.
NOTE: We do not recommend placing the bees in the refrigerator or freezer since it is difficult to monitor when the bees become immobile, resulting in excessive exposure to the cold. When harnessing more than 10 bees, transfer only a few bees to the ice-water slurry at a time.
- Collect workers from the entrance to the colony as they pause before departing from the colony or as they return from foraging.
- Restraining the Bees
- Place the bee in the harness with its dorsal thorax facing the cut-away portion of the tubing and its head just above the top of the tubing. Gently press the bee close to the tubing so its mouthparts extend beyond the edge of the tubing. Slide the strip of duct tape in between the head and the thorax on the dorsal side of the bee and firmly attach the free end to the side of the harness.
- Make sure the tape on the harness is smooth and taut. There should be no noticeable gaps between the front of the bee harness and the tape, and the tape should lay flat on the top of the harness. If there are gaps the bee may not be able to extend its proboscis properly or it may escape. Also, make sure the bee’s front legs are not protruding between the front of the harness and the duct tape.
- Place the bee in the harness with its dorsal thorax facing the cut-away portion of the tubing and its head just above the top of the tubing. Gently press the bee close to the tubing so its mouthparts extend beyond the edge of the tubing. Slide the strip of duct tape in between the head and the thorax on the dorsal side of the bee and firmly attach the free end to the side of the harness.
- Feeding the bees and the Feeding to Conditioning Interval
- About 30 min following harnessing – after the bees have recovered from the initial setup – feed the bees 3-4 μl 0.5 M sucrose (in water). This amount will be sufficient for a feeding-to-conditioning interval of 3-4 hr.
- In general, feed 1 μl 0.5 M sucrose for approximately each hour of wait time between feeding and conditioning to ensure the bees survive the interval yet are hungry enough at the beginning of conditioning to be motivated to learn the conditioned association. In a laboratory kept at a warm temperature or if using a different concentration of sucrose, adjust the amount fed or the time interval accordingly.
NOTE: With a feeding to conditioning interval of 24 hr, make sure all the bees are fed to satiation with 0.5 or 1 M sucrose at least 24 hr before conditioning will begin. At room temperature, bees require approximately 24 hr before they are motivated enough to respond well in a conditioning protocol.
NOTE: Use a lower concentration of sucrose solution for feeding the bees than the concentration used as a reward during conditioning to prevent the bees from becoming less sensitive to the concentration of sucrose used during conditioning, which may reduce learning performance18. - Feed the bees in an area well away from the training area. Exposure to the unconditioned stimulus (sucrose) in the conditioning context before conditioning might influence subsequent conditioning to the odor.
- In general, feed 1 μl 0.5 M sucrose for approximately each hour of wait time between feeding and conditioning to ensure the bees survive the interval yet are hungry enough at the beginning of conditioning to be motivated to learn the conditioned association. In a laboratory kept at a warm temperature or if using a different concentration of sucrose, adjust the amount fed or the time interval accordingly.
- During the interval between feeding and conditioning place the bees on the countertop in a quiet area of the lab to avoid unnecessary disturbance. If the laboratory has low ambient humidity, place bees in a plastic container with wet paper towels during the time interval. This prevents desiccation, since the bees may die if they are exposed to low humidity for prolonged time periods.
NOTE: Do not change the harnessing and feeding protocol within an experiment! Use only one specific time interval between setting up and feeding the bees and beginning conditioning for each experiment so the bees are all treated exactly the same way.
- About 30 min following harnessing – after the bees have recovered from the initial setup – feed the bees 3-4 μl 0.5 M sucrose (in water). This amount will be sufficient for a feeding-to-conditioning interval of 3-4 hr.
3. Conditioning
- Testing for Sucrose Sensitivity
- A few minutes prior to beginning conditioning, test the bees’ motivation to feed by touching their antennae with a small droplet of 0.5 M sucrose solution (the same concentration used during feeding). Do not allow them to feed during this test. If they respond by extending their proboscis, they are probably sufficiently motivated to learn and can be used for the protocol.
- Experiment Design
NOTE: This protocol can be modified to fit a vast array of learning protocols in order to investigate many aspects of honey bees’ learning and memory capabilities. Among the many parameters that can be adjusted are the: Inter-trial interval, number of bees trained at a given time, inter-stimulus interval, type and concentration and number of odors, type and concentration of the unconditioned stimulus, the number and sequence of trials (Figure 1; Table 1)19-21. When designing the exact protocol for each conditioning trial, it is very important to keep the inter-trial interval consistent across individuals. An inter trial interval that is too short, too long or inconsistent will impair the bees’ performance. See the discussion for a full treatment of this topic.
NOTE: Bees will learn the task more quickly if they are in a warm environment. Therefore, keep the temperature of the training environment consistent and warm (29-30 °C if possible). - Delivering the Odor (Conditioned Stimulus)
- Method 1
- Setting up the Odor Delivery System
- Mount the plastic 20 ml syringe loaded with the odor-treated filter paper so it is stable and the small end is facing directly at the bee’s antennae. Set the plunger at the 15 ml mark and position the syringe so the plunger is easily accessible.
- Presenting the Odor Stimulus
- At the beginning of each trial, place the bee in the harness on the peg in the conditioning arena with its antennae pointed directly toward the odor cartridge. Let the bee acclimate to the arena for 15–25 sec before beginning the odor stimulus to reduce disruption caused by the abrupt change in its visual surroundings during the odor stimulus.
- If the bee begins to respond to the odor cartridge immediately after it is placed in the conditioning arena, there may be odor on the cartridge’s tip. Remove the contaminating odor by washing the cartridge off with a lint-free tissue soaked in 70% ethanol. If that does not solve the problem, replace the cartridge.
- Press the plunger of the syringe at a steady rate so the plunger is completely depressed within 4 sec. Monitor the bee’s response to the odor before presenting the unconditioned stimulus.
- Following each trial, allow the bee to rest in the conditioning arena for another 15–25 sec. Moving the bee too soon following the trial will significantly reduce the effectiveness of each conditioning trial.
- At the beginning of each trial, place the bee in the harness on the peg in the conditioning arena with its antennae pointed directly toward the odor cartridge. Let the bee acclimate to the arena for 15–25 sec before beginning the odor stimulus to reduce disruption caused by the abrupt change in its visual surroundings during the odor stimulus.
- Setting up the Odor Delivery System
- Method 2
- Setting up the Odor Delivery System
- Airflow Source and Flow Rate
- As the source airflow for odor delivery use an aquarium aerator pump or a lab bench pressurized air supply, if the laboratory is equipped with it.
NOTE: A typical aquarium aerator pump will have an airflow rate near 400 ml/min (7 ml/sec); over the 4 sec odor stimulus, 28 ml of air will flow through a 1 ml cartridge. - Use a flowmeter in line with the tubing of the air delivery apparatus to regulate the rate of flow for the air supply. Adjust the flow meter until the rate of flow is 400 ml/min. Check the flow rate at the opening of the odor cartridge with a separate flowmeter.
- Connect the airflow to the odor cartridge through a system of plastic tubing and valves.
NOTE: The timing device discussed below opens the valves at the appropriate time in the trial. Plastic connectors attach the airflow system to the odor cartridge through a Luer type attachment to the cartridge’s syringe barrel. - Regardless of the airflow source, check the flow rate periodically to make sure the line is intact and the flow rate is maintained at the desired rate.
- As the source airflow for odor delivery use an aquarium aerator pump or a lab bench pressurized air supply, if the laboratory is equipped with it.
- Airflow Source and Flow Rate
- Automated Odor Delivery
- Use a programmable logic controller (PLC) to automate the delivery of the odor.
NOTE: The PLC is programmed to open the valves to the odor delivery air flow 6 sec after initiating the program (initiated by pushing a button), keep the valves open for 4 sec, and sound an audible feeding signal, via a small speaker, 3 sec after odor stimulus onset to signal the experimenter to deliver sucrose. - Use a small LED to indicate the timing of the odor stimulus during each trial. If possible use a red LED, since bees’ photoreceptors are shifted away from longer [red] wavelengths and toward shorter [uv] wavelengths so they cannot see it well. Position the LED behind and below the bee, outside of their visual field, so the light will not become an inadvertent conditioned stimulus.
NOTE: The light also helps verify that the PLC is operating properly and is useful in analyzing video recordings of the bees’ responses during testing trials.
- Use a programmable logic controller (PLC) to automate the delivery of the odor.
- The Exhaust System
- Set up an exhaust system behind the bee to help pull the air past the bee and to evacuate the odor-laden air from the conditioning arena to maintain a discrete timeframe for each presentation of the odor.
NOTE: If there is a vacuum system in the laboratory, modify the vacuum port and attach a conduit of dryer tubing to provide the airflow required to evacuate the conditioning arena. If there is not a vacuum system, a small electric fan housed in line with the dryer tubing led to a fume hood or through a window will suffice.
- Set up an exhaust system behind the bee to help pull the air past the bee and to evacuate the odor-laden air from the conditioning arena to maintain a discrete timeframe for each presentation of the odor.
- At the beginning of each day, check the electrical and air connections of the odor delivery system and the exhaust system. Press the start button on the odor delivery system and track the time between pressing the button and when the valves open (a quiet click and the LED light comes on) and when the cue to present the unconditioned stimulus (an audible tone) sounds. Check the air delivery by placing a moist fingertip in front of the connector that will be attached to the odor cartridge. There should be a strong air stream only when the valves are open. Check the exhaust system by holding a lint-free tissue up to the opening of the exhaust system to make sure the airflow is strong enough to properly evacuate the air from the conditioning arena.
- Setting up the Odor Delivery System
- Mounting the Odor Cartridge
- Place some modeling clay either on the countertop or on a small Plexiglas stand to position the bee and odor cartridge directly in front of the exhaust system. Place the glass odor syringe in the clay and adjust it so the opening in the wide end is pointed at the bee's head. The end of the syringe should be 1-2 cm from the bee. Pick a distance within this range and be constant.
- Securely place the fitting connecting the cartridge to the air tubing and valve system over the narrow ground glass end of the syringe.
- Presenting the Odor Stimulus
- At the beginning of each trial, place the harnessed bee on the peg in the conditioning arena with its antennae pointed directly toward the odor cartridge. Let the bee rest in the arena for 15–25 sec before beginning the odor stimulus to allow it to become accustomed to its new surroundings.
NOTE: If the bee begins to respond to the odor cartridge immediately after being placed in the conditioning arena, there may be odor on the cartridge’s tip. Remove the contaminating odor by washing the cartridge off with a lint-free tissue soaked in 70% ethanol. If that does not solve the problem, replace the cartridge. - Press the start button to initiate the timing mechanism for odor delivery. Monitor the bee’s response to the odor after stimulus onset and before presentation of the unconditioned stimulus.
- Following each trial, allow the bee to rest in the conditioning arena for another 15–25 sec to allow for initial memory formation. Moving the bee too soon following the trial will significantly reduce the effectiveness of each conditioning trial.
- At the beginning of each trial, place the harnessed bee on the peg in the conditioning arena with its antennae pointed directly toward the odor cartridge. Let the bee rest in the arena for 15–25 sec before beginning the odor stimulus to allow it to become accustomed to its new surroundings.
- Method 1
- Delivering the Sucrose (Unconditioned Stimulus)
- Method 1
- For this method, use a toothpick to deliver the sucrose.
NOTE: It is best to use a plastic toothpick since the wooden toothpicks can have an odor that will influence the bees’ responses to the conditioned stimulus. - Dip the tip of the toothpick into the sucrose solution, and, when it is time to present the unconditioned stimulus, hold the toothpick in front of the bee and allow the bee to lick the sucrose from the toothpick for approximately 1 sec.
- Replace the toothpick regularly to avoid buildup of sucrose.
- For this method, use a toothpick to deliver the sucrose.
- Method 2
- Sucrose Delivery System
NOTE: A more accurate way to deliver the unconditioned stimulus is through use of micrometer syringes, which can accurately deliver small amounts (tenths of a microliter) of the sucrose reward.- Wind the micrometer of the syringe back as far as possible. Load the micrometer syringe barrel with the sucrose solution. Make sure there are no bubbles in the glass portion and assemble the syringe. Fill the hub of the needle with the sucrose solution before placing it on the narrow end of the syringe barrel. To make sure the fluid in the needle is actually sucrose and not water from washing the needle, wind the micrometer forward 1–2 μl before beginning the experiment.
- Sucrose Delivery System
- Sucrose Presentation
- Hold the tip of the needle approximately 2 cm from the bee while the odor is presented. Use the edge of the training arena to prevent shaking the syringe as the movement can distract the bee. Do not get the needle too close in anticipation of the feeding signal, since that will also prematurely shift the bee’s attention from the odor to the sucrose.
- As soon as the feeding signal sounds, lightly touch the antennae until the bee extends its proboscis, then feed the bee. This should require only a very light touch or two to the antennae.
- Use a reward amount between 0.2 and 0.8 μl. The bee should easily consume the entire droplet within the time period allotted for the unconditioned stimulus (~1-2 sec). Do not use “ad libitum” feeding from a large droplet, which the bee feeds on for several seconds but cannot entirely consume.
- Watch for sucrose build up on the antenna, which will reduce the bee’s response to subsequent presentations of the reward. Modify the feeding procedure if this occurs.
- To ensure accurate pairing of the odor cue and the sucrose reward, make sure to present the droplet of sucrose solution to the bee as soon as possible after the tone.
NOTE: Timing between the onset of the CS (odor) and US (sucrose) is critical. Ideally, the odor presentation and sucrose presentation should briefly overlap. If more than a few seconds elapse between the end of the CS and delivery of the US, conditioning performance will diminish.
- Method 1
4. Testing
- Following conditioning use unreinforced test trials to assess how well the bees learned and/or remember the conditioned association.
NOTE: See the discussion for a detailed explanation of the importance of analyzing the bees’ responses to unreinforced test trials in addition to the bees’ performance in the conditioning trials and an explanation of the purpose of number of test trials and test odors. - Consolidation Time
- Following conditioning, either administer an immediate test trial or allow the bees to rest through a consolidation period.
NOTE: The length of the consolidation period, the time interval between conditioning and testing, will depend on the goal of the experiment. If the purpose is to investigate learning differences or short-term memory, the bees’ performance on test trials can be evaluated immediately following or several hours after conditioning. If the experiment involves investigating long-term memory, the test trials should be given at least 24 hr following the end of conditioning. - If the test trials are more than 24 hr following conditioning, feed the bees to satiation with 0.5 M sucrose following conditioning and at least once per day afterward until 12-24 hr before the test trials.
- To make sure the bees have reached satiation, feed each of them several different times until they no longer extend their proboscis in response to touching their antennae with sucrose. They can consume as much as 40 μl to reach satiation.
- Keep the bees in a humidified container to prevent their desiccating and dying during the consolidation period.
- Following conditioning, either administer an immediate test trial or allow the bees to rest through a consolidation period.
- Test Trials
- Use only newly prepared odor cartridges for testing.
NOTE: The cartridges used for acquisition may be differentially depleted, so the test stimulus will not be consistent across trials, odors, and individual bees and, therefore, could significantly affect the consistency and robustness of the results. - During testing, maintain the same inter-trial interval and other parameters as used in the conditioning phase of the experiment.
- Do not present the unconditioned stimulus during test trials, since the presence of a reward will alter the bees’ response to the conditioned stimulus, potentially masking more subtle effects of the conditioning protocol or a treatment.
- Record videos of all of the test trials to be able to later measure multiple aspects the bees’ responses to the odor(s).
- Use only newly prepared odor cartridges for testing.
5. Recording the Bees’ Responses
- Binary (Presence/Absence) Scoring of PER
- Use a binary scoring system of PER during conditioning trials and during testing trials. Score responses as either positive or negative; this is satisfactory for many kinds of analyses with a sample size of 20-40 bees per treatment group. The precise topology of responses can be complex22. It is important to establish an easy to see and score criterion for a positive response.
- Score a bee’s response as positive (+) when the bee extends its proboscis beyond the line made by connecting the tips of the opened mandibles. Only score a positive response when the bee extends its proboscis after odor onset but before odor offset.
- Score a bee’s response as negative (−) when there is no extension of the proboscis during the trial or if the proboscis is extended after the offset of the odorant.
- Avoid trying to score partial responses. To make finer distinctions in the bees’ behavior, use the video analysis technique outlined below.
- Use a binary scoring system of PER during conditioning trials and during testing trials. Score responses as either positive or negative; this is satisfactory for many kinds of analyses with a sample size of 20-40 bees per treatment group. The precise topology of responses can be complex22. It is important to establish an easy to see and score criterion for a positive response.
- Video Analysis of Additional PER Measurements (Movie 1)
- Gather additional information from behavior tests by analyzing videos of a bee’s responses to an odor.
NOTE: These measurements can also provide a higher temporal resolution for examination of the bees’ responses to the odors, particularly when there is not a difference in the presence/absence scoring of PER between treatment groups. - Recording the videos
- Always use unreinforced test or extinction trials for video recordings. The presence of the reward will alter the bees’ response to the odor, modifying the duration of proboscis extension.
- Set a video camera on a tripod above the training arena and focus the camera on the front of the bee’s head, so the antennae and proboscis are in sharp focus.
- Position the small LED light, which indicates odor stimulus presentation, in an area behind the bee that is visible in the video in order to use it to identify odor onset and offset during analysis.
- Begin recording 20 sec before odor onset and continue recording for at least 20 sec after odor offset before disturbing the bee.
- Video Analysis
- Upload the videos into video editing software, such as iMovie or Final Cut Pro, with the capability of measuring time intervals between specific frames in the video.
- Analyze the bees’ response to the odor for a discrete time period beginning at the onset of the odor stimulus (e.g., 10 or 20 sec).
- Use the following common measurements for evaluating PER:
- Measure the duration of proboscis extension, which is the total time that the proboscis extends beyond the mandible-to-mandible line (described above).
NOTE: A bee may extend and retract its proboscis multiple times during the 10-20 sec time window. Measure each proboscis extension individually, and calculate the total duration. Analyzing the number and duration of each individual proboscis extension can also provide valuable information. - Measure the latency of proboscis extension, which is the time between odor onset, when the LED light comes on, and the first proboscis extension.
- Count and time Glossal extensions relative to the onset of odorant, the first extension, or other timing parameters.
- Track Antennal movements23, which also show characteristic odor-oriented changes.
NOTE: In Movie 1, the antennal movements have been tracked with video tracking software to illustrate the change in antennal movements in response to an odor stimulus.
- Measure the duration of proboscis extension, which is the total time that the proboscis extends beyond the mandible-to-mandible line (described above).
- Gather additional information from behavior tests by analyzing videos of a bee’s responses to an odor.
Representative Results
We present two examples of use of the PER protocols described above. The first example made use of Method 2 to study how honey bees perceive different odors as a function of the molecular similarity to odor used as the CS5,24,25. The second is an example of the use of Method 1 and some of the precautions that must be taken when beginning to use PER conditioning experiments in the research laboratory.
Studies such as this one have been used to describe the olfactory ‘perceptual space’ of honey bees and moths in conjunction with bioimaging13 or electrophysiological analyses26. Honey bees (n = 20) were conditioned to associate the odorant decanal with sucrose reinforcement over 12 forward pairing conditioning trials (Figure 3A). Approximately 10% of the honey bees responded ‘spontaneously’ on the first trial, which is normal. After that the percentage that responded increase over the next few trials until 100% of the honey bees responded on the fifth trial and every subsequent trial. Several studies have shown that this increase in response is specific to the forward pairing condition (Figure 1) relative to several important control procedures3. After the acquisition phase, each honey bee was subjected to a series of unreinforced test trials (Figure 3B), which involved exposure to the odor CS and to several other odorants that systematically differed from the CS in molecular structures. Honey bees responded most strongly to the CS. Their responses to the other odorants decreased as a function of the systematic changes in structure, with the lowest responses to the odorants least like the CS.
In Figure 4, the results from a recent workshop in which students learned, for the first time, how to condition honey bees using Method 1 are illustrated. Students first conditioned honey bees by forward pairing methycyclohexanone (MCH) or octanol (OCT) with sucrose reinforcement (Figure 4A). Percent responses were lower on the first trial and increased on subsequent trials, indicating that the bees learned the odor-sucrose association. There were differences in the degree that the bees learned the conditioned association across the different groups of students. In our experience, these kinds of differences could be due to the odor that was used as the CS, to the imprecise elements in the method (Method 1), or to the training level of the experimenters. In the third case, performance quickly becomes more consistent with the experimenters’ increased experience. After conditioning, each honey bee was tested twice with each odor. In groups of honey bees that had been conditioned with forward pairing of OCT or MCH, the response was, as expected, strongest to the conditioned odor (Figure 4B).
The second group of honey bees that experienced forward pairing to OCT was conditioned in parallel to another group in which the odor OCT was backward paired with sucrose. Backward pairing is one of several types of control procedures to demonstrate that the increase in response is specific to forward pairing and not due to another process, such as non-specific arousal from sensitization. For backward pairing, the sucrose US and odor CS are presented in reverse order to what is shown in Figure 1. Acquisition data from backward pairing are not shown because the CR cannot be scored given the presentation of the odor CS after presentation of sucrose. As would be predicted for associative conditioning, the response levels to OCT were higher in the forward paired group relative to the backward group (Figure 4C).
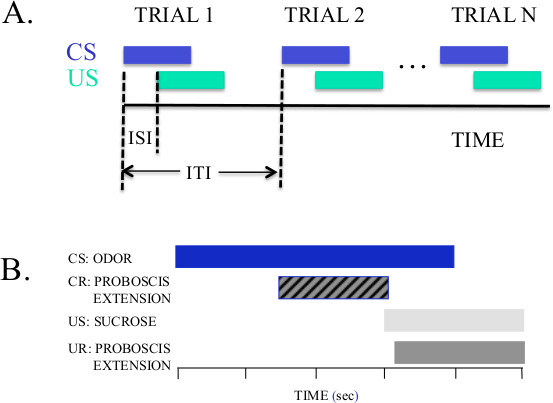
Figure 1. Diagrams of experimental design for conditioning using forward pairing. See Table 1 for definitions of the terms used in this figure. (A) The CS (odor) precedes and overlaps with the US (sucrose/water solution). The relationship between the CS and US shown is optimal for conditioning honey bees and moths. But the optimal ISI can depend on the conditioning protocol and animal species1. (B) Breakdown and relative timing of CS, CR, US, and UR. The CR that occurs either before US presentation or during unreinforced ‘test’ trials is the dependent measure for experimental protocols.
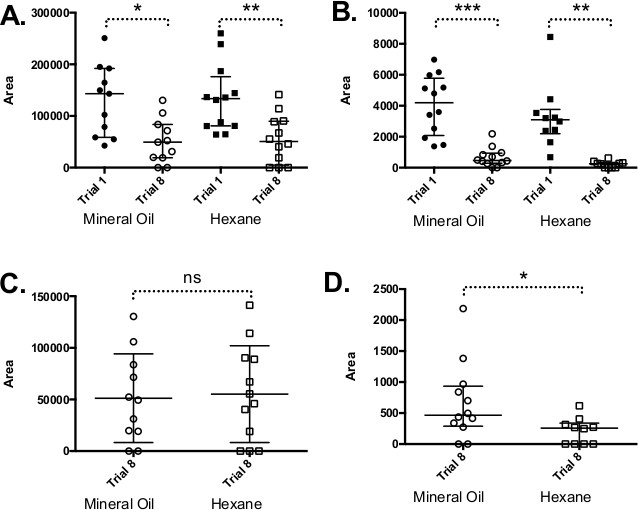
Figure 2. Odor depletion with reuse of cartridges. For these data, odor cartridges were set-up with hexanol as described in the text. Hexanol is commonly used as a CS in PER studies; however, the actual rate of depletion will depend on the odor and solvent. Cartridges were then used once for 4 sec every minute over 8 trials (assuming 5 honey bees/trial in a standard experiment that would equal 40 uses). The dilutions in the indicated solvent, which were relatively standard for PER experiments, were 2.0 M (A) and 0.2 M (B). (A, B)The odor was sampled after the first and the eighth trials by adsorption onto a Solid Phase Microextraction (SPME) fiber that was then desorbed onto a gas chromatograph. The relative areas under the peaks were higher after the first trial relative to the eighth for both solvents and for both concentrations (Wilcoxon matched pairs signed rank test p < 0.05 [*] or greater), which shows that less odor was delivered from the cartridge after the cartridge had been used a number of times. (C, D) The same data showing just the eighth trial with the y-axis expanded. It shows that depletion was slightly greater for hexane relative to mineral oil, but only at the lower concentration.
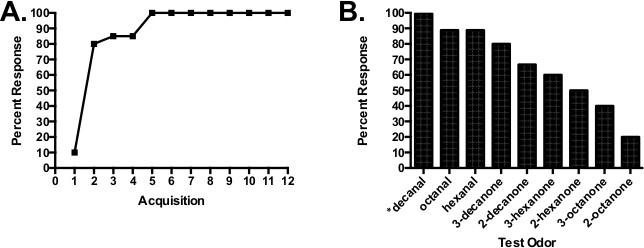
Figure 3. Acquisition and test using decanal as the CS and method 2 to conditioning. (A) Trial-by-trial response (CR) over 12 forward pairing trials (Figure 1). Data are from n = 20 honey bees conditioned in four groups of 5 honey bees each. The ISI was 3 sec and the ITI was 6 min. (B) Unreinforced tests using odors that differ from decanal by carbon chain length and/or position of the carbonyl carbon. The odors were presented in a randomized series across the four groups and were interspersed with reinforced trials with decanal (every two to three trials) in order to avoid extinction of the CR.
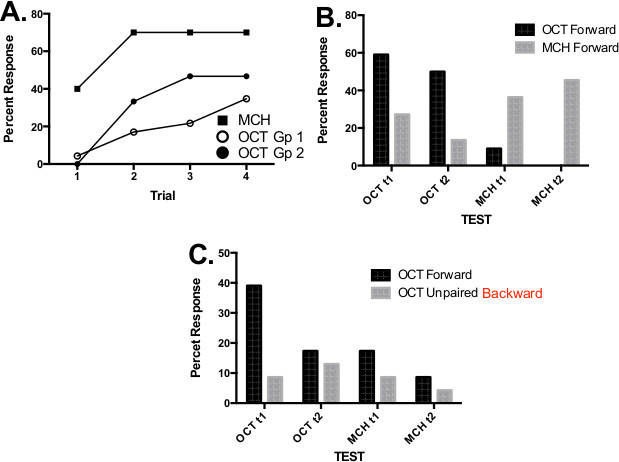
Figure 4. Data from a workshop during which graduate students were trained to condition honey bees using PER method 1. (A) Acquisition data from three groups of honey bees conditioned to octanol (n = 23 and 15 for Gps 1 and 2, respectively) and methylcyclohexanone (n = 10). (B) CRs during unreinforced tests with both odors; four trials presented in random series across groups. (C) Unreinforced tests with both odors in two groups of honey bees, one forward paired (Gp 1, n = 23) and one backward paired (n = 25).
| Term (abbreviation) | Definition | Relevant Example |
| Conditioned Stimulus (CS) | A stimulus that elicits little or no response at first and signals US | ODOR |
| Conditioned Response (CR)a | The response to the CS after association with the US | PROBOSCIS EXTENSION |
| Unconditioned stimulus (US) | A biologically significant stimulus that elicits a response | SUCROSE/WATER SOLUTION |
| Unconditioned Response (UR)a | The response to the US | PROBOSCIS EXTENSION |
| Inter-Stimulus Interval (ISI) | The time between onset of the CS and onset of the US | ODOR to SUCROSE interval |
| Inter-Trial Interval (ITI) | The time between successive CS-US pairings for a single animal | ODOR (trial n) to ODOR (trial n+1) interval |
Table 1. Important Terminology. Refer to Figure 1 for an illustration of these terms. aFor PER conditioning the CR and UR are the same, although for other types of associations and reflexes the CR and UR may differ.
Movie 1. A video of a honey bee performing PER after it had been conditioned to respond to an odor. Note the PER response of the bee approximately 1.5 sec following odor onset. Tracking software mapped the antennal movements in response to presentation of the odor. The lines plotted in the upper right hand corner of the video depict the actual antennal movements. The two graphs at the bottom of the screen show antennal movements on the X (left) and Y (right) coordinates. The vertical lines in the graph shown in the video indicate, left to right, light ON, PER, light off and proboscis retraction.
Discussion
This protocol presented two reliable methods for conditioning using the PER procedure. These are two of several methods that have been successfully employed27,21. We employ Method 2 for all experimental studies using PER as it is consistently reliable, even across different experimenters.
The same basic procedure has been adapted to many different kinds of studies with honey bees, including use of different conditioning stimuli and different behavioral reflexes. It has also been linked to investigations of the genetic basis of differences in learning28,29, the physiological correlates of olfactory perception and memory in the brain13,14,30, and the modulatory and molecular genetic bases of behavior15,16,31. Because of the advanced knowledge of the ecological relevance of olfactory learning to honey bees, which started with the first studies by Karl von Frisch over 100 years ago31, results from PER can be easily linked to survival needs of a colony. Most recently, it has been adapted to an agricultural study to investigate the sublethal behavioral effects of pesticides and environmental toxins17.
The basic procedure is powerful in that it can be applied to investigate problems in other species as well. Two moth species, Manduca sexta and Spodoptera littoralis, have been used in PER studies to investigate the neural basis of odor identification and each species’ olfactory learning capabilities32,33. PER experiments with fruit flies have provided many insights into the molecular signaling cascades involved in chemosensation and learning34. And PER has recently been used to study habituation in the flea (Rhodnius prolixus)35, an important disease vector.
In general, the procedure is robust to changes in method; the use of different methods will likely produce the same relative difference between treatment groups. In spite of the relatively simple procedure, several problems can at times prevent honey bees from learning the odor-sucrose association. The following topics are possible alterations to the protocol and some of the more common problems that may arise during PER conditioning.
Considerations on Number and Type of Trials in the Conditioning Protocol
Any learning protocol will require exposure of the bees to a number of trials in the acquisition phase. This number depends largely on the difficulty of the task. Honey bees can learn a simple task after a single trial, but they need at least a three trials to induce long-term memory formation. As expected, the honey bees will require significantly more trials to adequately learn a difficult task. Generally, there is a maximum number of trials beyond which the bees no longer significantly improve their performance. This maximum depends on the specific task, the odor types and concentrations, and the sucrose concentration.
When using more than one type of trial with different odors, present the different trial types in a pseudorandom sequence to keep the bees from memorizing a simple sequence of odor presentations instead of learning the differences between the odors36. In these pseudorandom sequences, there must be an equal number of trials for each of the odor types. Also, the probability of a trial of one odor type being preceded by and followed by the same odor, or any of the other odors, must be equal for all the odors. For two odors – A and B – use the following sequence: ABBABAAB. Over eight trials each odor is presented four times. Concatenate identical sequences to reach the desired number of trials for each odor.
Regardless of the experimental design, there are a few parameters that must remain constant in order to optimize learning. The overlap between the CS and the US is critical for effective conditioning. The inter-trial interval (Figure 1, Table 1) needs to be constant and be optimized because irregularity or a too short or too long inter-trial interval can significantly influence the effectiveness of the conditioning protocol1.
Considerations on the Importance and Design of Behavioral Testing Trials
Responses recorded during the ‘acquisition phase’ of an experiment, when the CS and US are presented together, can be useful. However, beware that the odor presentation conditions can differ from one type of trial to another. During reinforced trials, the odor followed by sucrose is presented in conjunction with a visual stimulus (movement of the device for delivering the sucrose droplet) that can affect the bee’s response. And the bees have only three seconds to show a response (four seconds of odor stimulation minus the one sec overlap with sucrose presentation; Figure 1). If the experiment involves the bees learning to differentiate two odors (e.g., reinforced and unreinforced), the unreinforced odor presented on alternate trials occurs without the visual stimulus of the reward presentation and the bees have the full four seconds to respond. Therefore, the responses to the two odors are not fully comparable during acquisition. With any conditioning protocol, do not rely solely on acquisition curves1. To better ascertain what bees have learned, introduce a series of unreinforced test trials, during which neither odor is reinforced, which ensures that testing of all stimuli takes place under identical conditions.
Depending on the specific purpose of the experiment, testing can consist of single test trials of the conditioned odor or a series of trials with the conditioned odor or a combination of the conditioned and novel odors. A single test trial of the conditioned odor provides a simple assay of whether the bees’ remember the conditioned odor. However, the response to the first test trial may reflect the bees’ motivation level as well as their recall of the conditioned odor. A series of test trials, either a series of extinction trials of the conditioned odor or a series of single tests of the conditioned and one or more novel odors, can also be used to assess memory. The series of extinction trials can assess how strongly the bees’ remember the conditioned association. The stronger the recall the greater number of trials needed to extinguish the conditioned response. A series of single tests of conditioned and novel odors can also assess the specificity the bees’ memory of the conditioned odor.
It is also imperative to condition and test both control and treated groups at the same time points. Comparing the performance of bees soon after conditioning to bees held for longer time periods is problematic because of the exposure to the sucrose US by feeding the bees to satiation. For example, a decrease in performance after long intervals could be due to memory decay or it could be due to changes in motivational state and/or learning induced by unreinforced exposure to the US, making the results ambiguous. Therefore, performance of a treatment group should always be judged relative to a control group conditioned and tested at the same time points.
Odor Concentration and Integrity
There are several ways in which the concentration and integrity of the odor (CS) can be compromised. The most prevalent problem with odor delivery is the depletion of odor cartridges from overuse (Figure 2). Replace the cartridges 10-12 uses (every 2 or 3 trials with groups of 5 honey bees) to avoid this pitfall. It is also critical to use fresh cartridges for test trials, since used cartridges may be differentially depleted and thus present unequal odor stimuli. Another common problem is odor cartridge contamination due to using the cartridge for more than one odor without completely cleaning it. A dirty or contaminated air flow can also unintentionally introduce additional olfactory stimulus (activated charcoal filters can prevent background contamination). This is especially problematic when coupling PER conditioning with measurements of the physiological responses to the odor. Leaky odor cartridges present a problem since the honey bees are exposed to odor before the trial begins, which reduces the salience of the odor stimulus. Loose fitting on the air supply for odor delivery can result in little or no odor delivery when the valve opens, artificially reducing the bees’ response to the intended odor.
Sucrose Solution and Unconditioned Stimulus (Reward)
The amount, concentration, and integrity of the sucrose solution used as the US are vital to the success of the experiment. Conditioning is a function of the amount and concentration of sucrose-water solution used as the US37. The micrometer syringes used in Method 2 allow for very precise (down to 0.2 μl) control of US delivery, and we recommend using them for both methods described. Use of the toothpicks (Method 1) is adequate for circumstances in which the expensive syringes cannot be used, such as, in training large groups of students, work in the field, or with lower budgets. Careful implementation of Method 1 is fine as long as timing is maintained with regular replacement of toothpicks to avoid buildup of sucrose (and the concentration delivered). However, using toothpicks it is more difficult to accurately control and estimate the amount and magnitude of the US delivered, which is important for conditioning studies1. The concentration of sucrose needed to provide a sufficient reward to keep the bees motivated to learn the conditioned association can depend on the difficulty of the task and the bees’ internal state or time of year. The more difficult tasks require a higher sucrose solution for the bees’ to successfully learn the task. Mold can buildup in sucrose solutions even at 5 °C, which will compromise the integrity of the solution, affecting the bees’ health and perception of the reward during the experiment. It is best to replace the solution every few days.
Precision, Timing, and Consistency of CS and US Delivery
The most critical issue about proper implementation of a PER procedure, or for that matter any conditioning procedure, involves precision, timing and consistency of CS and US delivery (Figure 1). Investigators who are new to the procedure frequently are imprecise about delivery of one or both stimuli. ISI’s that fail to allow for overlap the CS and US result in poor conditioning performance. The PLC automates an audible signal for the experimenter to deliver sucrose 3 sec after the onset of odor delivery. Investigators should hold the sucrose/water droplet close to the bee for rapid delivery after the signal. These procedures help entrain a consistent ISI. Placing a stopwatch by the conditioning arena allows for convenient time placement of the trial as well as monitoring the bees’ time in the arena both before and after delivery of stimuli. That way the ITI’s can be relatively consistent and the entire procedure can be run at a controlled pace. ITI’s that are too short, for example less than 1 min, or too long can lead to poor conditioning performance1.
Seasonal, environmental, and Contextual Effects on Honey Bee Performance
Honey bees’ performance can be significantly influenced by its surroundings both prior to and during conditioning. Frequently, the fluctuations in temperature and food availability that come with the changing seasons will alter the bees’ motivation to learn. When flowers are in bloom, the bees’ motivation to learn the conditioned association in the lab decreases38. When the colony is stressed – from extreme temperatures, food shortages, or disease-related stress – the bees’ will show a reduction in their learning performance inside. Honey bees kept in a flight room may learn well for a while, but there too the stress of disease and aging degrade their learning performance over time. The context during conditioning can also decrease the bees’ performance. Any extraneous odors, movement, and other stimuli can distract the bee from the experimental stimuli. To avoid this problem, maintain a reasonably consistent, simplified visual context.
Genotype and Experience Affects PER Performance
Honey bee workers can differ considerably in performance on any conditioning procedure due to task specialization, genotype, or other environmental factors20. Therefore, it is important to standardize, as much as possible, the types of animals used in an experiment in order to reduce inter-individual variation. In a colony led by an open-mated queen, which means she mated with many different drones, the workers will differ in paternal genotype. Genetic background can lead to dramatic differences in sensory responsiveness39 and learning performance29. Using colonies led by queens instrumentally inseminated by sperm from single drones28 reduces this inter-individual variation.
The protocols described above, includes a method for collecting honey bee workers from the nest entrance. However, these honey bees differ from each other in regard to age or behavioral task. They may be young (inexperienced) or older (more experienced) foragers. They may be young honey bees making their first orientation flights. Or they may be guard bees. To reduce the variability, mark the bees with a quick-drying enamel paint or marking tags either as they emerge as adults (to control for age) and/or as they begin foraging (to control for experience). Then, a few days later, collect the marked bees for conditioning. Workers that are engaged in nursing behavior can be collected from the frames within the hive. Nurses can be positively identified when they insert their heads into a brood cell to feed and care for the larva inside.
Using Virgin Queens or Drones for PER Conditioning
In addition to worker honey bees, virgin honey bee queens and drones can be readily conditioned in a PER procedure for studies aimed at developing genetic lines of honey bees that differ in learning performance28. Virgin queens should be collected soon after they emerge from pupation and placed directly into restraining harnesses without anesthetization. Young, immature drones collected from the brood comb inside the colony are generally not motivated to learn. After they have begun mating flights, drones readily learn PER tasks28. They should be collected as they return from a mating flight and maintained overnight in a small cage in a colony used for rearing queens. Do not attempt to harness them the day before conditioning; they do not survive well in harnesses overnight. A couple of hours prior to conditioning the drones can be collected from the cages and placed into the restraining harnesses without anesthetization.
Conclusions
This PER procedure, in the way of methods, amounts to a starting point for designing PER experiments. Most PER protocols will require that the procedure outlined be changed in some way to implement the specific goals of the experiment and accommodate multiple treatment groups. It is easy to implement. However, proper implementation requires attention to detail and practice. Once mastered it can be a powerful procedure to add as a research tool for several basic and applied research programs with different insect species.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by funding from NIH NCRR (R01 RR014166 to BHS), NIH NIDCD (R01 DC011422 BHS co-PI), the US Department of Agriculture (J Trumble PI; BH Smith co-PI) and Arizona State University. Funding for the workshop to train students (data in Figure 4) was provided by the science foundation of Chile.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Sucrose | Sigma-Aldrich | S9378-1KG | |
| Odorant compounds (for example): | For additional examples of odorants, see any of the papers on olfactory processing from the Smith lab | ||
| 1-hexanol | Sigma-Aldrich | 471402-100ML | |
| 2-octanone | Sigma-Aldrich | W280208-800G-K | |
| heptanol | Sigma-Aldrich | 51778-5ML | |
| gerianol | Sigma-Aldrich | 163333-25G | |
| nonanal | Sigma-Aldrich | 131210-100ML | |
| Hexane | Sigma-Aldrich | 296090-1L | |
| Heavy Mineral Oil | Sigma-Aldrich | 330760-1L | Make sure it’s odorless |
| Ethanol | Sigma-Aldrich | 459836-1L | |
| Scintillation vials | Sigma-Aldrich | Z190535-1PAK | Use a small drill bit to bore a small hole in the cap of the vials |
| Bee harness | Custom-made from 0.9 cm diameter plastic soda straws or hard plastic/metal tubing | ||
| Duct tape | |||
| Kimwipes | Sigma-Aldrich | Z188956-1PAK | |
| Wash bottles | Sigma-Aldrich | Z560847-3EA | For the 70% ethanol |
| Small fan in mountable housing | |||
| Dryer tubing | |||
| FOR METHOD 1 ONLY | |||
| Name | Company | Catalog Number | Comments |
| 20 ml disposable plastic syringes | Cole-Parmer | WU-07945-18 | |
| 15 mm filter paper circles | Sigma-Aldrich | Z274844-1PAK | |
| Pushpins | |||
| Toothpicks | |||
| FOR METHOD 2 ONLY | |||
| Name | Company | Catalog Number | Comments |
| Gilmont Micrometer syringe, 0.2 mL | Cole-Parmer | EW-07840-00 | |
| Gilmont micrometer syringe tip | Cole-Parmer | EW-07841-00 | |
| 26G 3/8” Leur hub hypodermic needles | Fisher Scientific | 14-826-10 | |
| 1cc tuberculin syringes (plastic/glass) | Sigma-Aldrich | Z181641-1EA OR Z192090-200EA | glass tuberculin syringes are available, but plastic syringes are much less expensive and will work well for a limited number of uses |
| Small rubber/silicone restrictors | Cole-Parmer | EW-95702-02 | Made from 4.8 mm outer diameter silicone tubing or the rubber tips of the 1 cc syringe plungers |
| Parafilm | Sigma-Aldrich | P7793-1EA | |
| 75 X 100 mm filter paper | Sigma-Aldrich | Z695106-500EA | |
| Direct Logic 05 Programmable Logic Controller | Koyo Electronics Industries Co, Ltd | http://www.koyoele.co.jp/ OR english/support/dlc.html#plc | |
| 1 mm, 4mm & 6 mm inner diameter PVC or silicone tubing | Cole-Parmer | Various | Cole-Parmer has a wide selection of suitable tubing |
| Polypropylene connectors & leur fittings | Cole-Parmer | Various | Cole-Parmer has a wide selection of connectors and fittings for many tube sizes |
| 65-mm Correlated Flowmeter | Cole-Parmer | EW-03216-08 | Aluminum with Glass float; For Liquids and Gases, With Valve |
| OR | |||
| Tetra Whisper 300 (Tetratek DW96-2) Aquarium Air Pump | Aquacave | AE-TETRA-300 | www.aquacave.com |
| LIF series Solenoid Valves for .042 " ID Tubing, Configuration "E" | The Lee Company | LFAA1200118H | Neoprene, 430 SS, 302 SS, 280mWatts http://www.theleeco.com/LEEWEB2.NSF |
| PC-Board 12VDC 70dB Piezo Buzzer | RadioShack | 273-074 | www.radioshack.com |
References
- Rescorla, R. A. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 11, 329-352 (1988).
- Martin, S. J., Grimwood, P. D., Morris, R. G. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 23, 649-711 (2000).
- Bitterman, M. E., Menzel, R., Fietz, A., Schafer, S. Classical conditioning of proboscis extension in honeybees (Apis mellifera). Journal of Comparative Psychology. 97, 107-119 (1983).
- Giurfa, M., Sandoz, J. C. Invertebrate learning and memory: Fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn Mem. 19, 54-66 (2012).
- Smith, B. H., Wright, G. A., Daly, K. S., Dudareva, N., Pichersky, E. . The Biology of Floral Scents. , 263-295 (2006).
- Menzel, R., Giurfa, M. Dimensions of cognition in an insect, the honeybee. Behav Cogn Neurosci Rev. 5, 24-40 (2006).
- Kuwabara, M. Bildung des bedingten Reflexes von Pavlovs Typus bei der Honigbiene, Apis mellifera. Journal of the Faculty of Science, Hokkaido University, Zoology. 13, 458-464 (1957).
- Menzel, R. The honeybee as a model for understanding the basis of cognition. Nat Rev Neurosci. 13, 758-768 (2012).
- Menzel, R., Kesner, R. P., Olton, D. S. . Neurobiology of comparative cognition. , 237-292 (1990).
- Hammer, M., Menzel, R. Learning and memory in the honeybee. Journal of Neuroscience. 15, 1617-1630 (1995).
- Strube-Bloss, M. F., Nawrot, M. P., Menzel, R. Mushroom body output neurons encode odor-reward associations. J Neurosci. 31, 3129-3140 (2011).
- Szyszka, P., Galkin, A., Menzel, R. Associative and non-associative plasticity in kenyon cells of the honeybee mushroom body. Front Syst Neurosci. 2, (2008).
- Fernandez, P. C., Locatelli, F. F., Person-Rennell, N., Deleo, G., Smith, B. H. Associative conditioning tunes transient dynamics of early olfactory processing. J Neurosci. 29, 10191-10202 (2009).
- Locatelli, F. F., et al. Nonassociative plasticity alters competitive interactions among mixture components in early olfactory processing. Eur J Neurosci. 37, 63-79 (2013).
- Farooqui, T., Robinson, K., Vaessin, H., Smith, B. H. Modulation of early olfactory processing by an octopaminergic reinforcement pathway in the honeybee. Journal of Neuroscience. 23, 5370-5380 (2003).
- Mussig, L., et al. Acute disruption of the NMDA receptor subunit NR1 in the honeybee brain selectively impairs memory formation. J Neurosci. 30, 7817-7825 (2010).
- Hladun, K. R., Smith, B. H., Mustard, J. A., Morton, R. R., Trumble, J. T. Selenium toxicity to honey bee (Apis mellifera L.) pollinators: effects on behaviors and survival. PLoS One. 7, e34137 (2012).
- Wiegmann, D. D., Smith, B. H. . International Journal of Comparative Psychology. 22, 141-152 (2009).
- Drezner-Levy, T., Shafir, S. Parameters of variable reward distributions that affect risk sensitivity of honey bees. J Exp Biol. 210, 269-277 (2007).
- Drezner-Levy, T., Smith, B. H., Shafir, S. The effect of foraging specialization on various learning tasks in the honey bee (Apis mellifera). Behavioral Ecology & Sociobiology. 64, 135-148 (2009).
- Shafir, S., Menda, G., Smith, B. H. Caste-specific differences in risk sensitivity in honeybees, Apis mellifera. Animal Behaviour. 69, 859-868 (2005).
- Smith, B. H., Menzel, R. An analysis of variability in the feeding motor program of the honey bee; the role of learning in releasing a modal action pattern. Ethology. 82, 68-81 (1989).
- Erber, J., Pribbenow, B., Kisch, J., Faensen, D. Operant conditioning of antennal muscle activity in the honey bee (Apis mellifera L). Journal of Comparative Physiology A-Sensory Neural & Behavioral Physiology. 186, 557-565 (2000).
- Smith, B. H., Menzel, R. The use of electromyogram recordings to quantify odorant discrimination in the honey bee, Apis mellifera. Journal of Insect Physiology. 35, 369-375 (1989).
- Stopfer, M., Bhagavan, S., Smith, B. H., Laurent, G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 390, 70-74 (1997).
- Daly, K. C., Christensen, T. A., Lei, H., Smith, B. H., Hildebrand, J. G. Learning modulates the ensemble representations for odors in primary olfactory networks. Proc Natl Acad Sci U S A. 101, 10476-10481 (2004).
- Matsumoto, Y., Menzel, R., Sandoz, J. C., Giurfa, M. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J Neurosci Methods. 211, 159-167 (2012).
- Chandra, S. B., Hosler, J. S., Smith, B. H. Heritable variation for latent inhibition and its correlation with reversal learning in honeybees (Apis mellifera). Journal of Comparative Psychology. 114, 86-97 (2000).
- Chandra, S. B., Hunt, G. J., Cobey, S., Smith, B. H. Quantitative trait loci associated with reversal learning and latent inhibition in honeybees (Apis mellifera). Behavior Genetics. 31, 275-285 (2001).
- Hammer, M. The neural basis of associative reward learning in honeybees. Trends in Neurosciences. 20, 245-252 (1997).
- Frisch, K. . The Dance Language and Orientation of Bees. , (1965).
- Daly, K. C., Smith, B. H. Associative olfactory learning in the moth Manduca sexta. J Exp Biol. 203, 2025-2038 (2000).
- Fan, R. J., Anderson, P., Hansson, B. Behavioural analysis of olfactory conditioning in the moth spodoptera littoralis (Boisd.) (Lepidoptera: noctuidae). J Exp Biol. 200. 23 (Pt 23), 2969-2976 (1997).
- Paranjpe, P., Rodrigues, V., VijayRaghavan, K., Ramaswami, M. Gustatory habituation in Drosophila relies on rutabaga (adenylate cyclase)-dependent plasticity of GABAergic inhibitory neurons. Learn Mem. 19, 627-635 (2012).
- Vinauger, C., Lallement, H., Lazzari, C. R. Learning and memory in Rhodnius prolixus: habituation and aversive operant conditioning of the proboscis extension response. J Exp Biol. 216, 892-900 (2013).
- Smith, B. H., Abramson, C. I., Tobin, T. R. Conditional withholding of proboscis extension in honeybees (Apis mellifera) during discriminative punishment. Journal of Comparative Psychology. 105, 345-356 (1991).
- Smith, B. H. An analysis of blocking in odorant mixtures: an increase but not a decrease in intensity of reinforcement produces unblocking. Behav Neurosci. 111, 57-69 (1997).
- Gerber, B., et al. Honey bees transfer olfactory memories established during flower visits to a proboscis extension paradigm in the laboratory. Animal Behaviour. 52, 1079-1085 (1996).
- Rueppell, O., et al. The genetic architecture of sucrose responsiveness in the honeybee (Apis mellifera L). Genetics. 172, 243-251 (2006).

