Fabrication and Visualization of Capillary Bridges in Slit Pore Geometry
Summary
A procedure for creating and imaging capillary bridges in slit-pore geometry is presented. The creation of capillary bridges relies on the formation of pillars to provide a directional physical and chemical heterogeneity to pin the fluid. Capillary bridges are formed and manipulated using microstages and visualized using a CCD camera.
Abstract
A procedure for creating and imaging capillary bridges in slit-pore geometry is presented. High aspect ratio hydrophobic pillars are fabricated and functionalized to render their top surfaces hydrophilic. The combination of a physical feature (the pillar) with a chemical boundary (the hydrophilic film on the top of the pillar) provides both a physical and chemical heterogeneity that pins the triple contact line, a necessary feature to create stable long but narrow capillary bridges. The substrates with the pillars are attached to glass slides and secured into custom holders. The holders are then mounted onto four axis microstages and positioned such that the pillars are parallel and facing each other. The capillary bridges are formed by introducing a fluid in the gap between the two substrates once the separation between the facing pillars has been reduced to a few hundred micrometers. The custom microstage is then employed to vary the height of the capillary bridge. A CCD camera is positioned to image either the length or the width of the capillary bridge to characterize the morphology of the fluid interface. Pillars with widths down to 250 µm and lengths up to 70 mm were fabricated with this method, leading to capillary bridges with aspect ratios (length/width) of over 1001.
Introduction
The study of the shape and resulting forces caused by capillary bridges has been the subject of extensive studies2-7. Initially most efforts were focused, due to their simplicity, on axisymmetric capillary bridges. Often capillary bridges occurring in natural systems, such as those found in granular and porous media8,9 and bridges employed in technological applications, such as for capillary self-assembly in flip chip technologies10-15 are asymmetric with nonuniform wetting properties on the interacting surfaces. The combination of improved lithography techniques along with the accessibility of simple numerical tools to model fluid interfaces allows for the creation and modeling of capillary bridges with increasing complexity.
Capillary bridges in slit-pore geometry offer an interesting compromise: the directional wetting properties lead to nonaxisymmetric bridges that retain some symmetry planes (which simplifies the analysis). They have been studied theoretically and numerically as a case study for porous media. Systematic experimental studies of capillary bridges in slit-pore geometry have, however, been limited. Here we present a method to create and characterize capillary bridges in slit pore geometry. Briefly, the method consists of 1) the fabrication of pillars to create a chemical and physical heterogeneity, 2) the design of a microstage to align and manipulate the bridges, and 3) the imaging of the capillary bridges either from the front or the sides to characterize their morphology. The characterization of the bridge morphology, along with comparisons to surface evolver simulations are provided in a separate publication1.
Protocol
The protocol text is broken up into three main sections: 1) the fabrication of the of the PDMS (polydimethylsiloxane) pillars, 2) the functionalization of the tops of the pillars, and 3) the formation and characterization of the capillary bridges.
1. Fabrication of the PDMS Pillars
This section details the fabrication of the PDMS pillars using die casting with a silicon/SU-8 mold.
- Fabrication of silicon/SU-8 mold
- Place a clean 4 in silicon wafer in a Pyrex Petri dish.
- Prepare a 4:1 (by volume) sulfuric acid to hydrogen peroxide (piranha) solution in a separate beaker.
Note: Extreme caution is needed in the preparation and use of the piranha solution. The reaction is highly exothermic and insulated gloves will be required to handle beakers. Piranha reacts violently with organics. Let piranha solution cool to room temperature before disposing. Only prepare enough solution required to submerge the wafer in the dish. - Pour piranha solution slowly onto the silicon wafer until it is completely submerged. Let sit for 15 min.
- Remove the wafer from the Petri dish and rinse under a stream of: deionized (DI) water for 2 min, ethanol for 30 sec, acetone for 30 sec, then blow dry with nitrogen.
Note: If residues from acetone are a problem, an additional rinse with IPA is recommended - Dry the wafer on a hot plate at 150 °C for 15 min.
- Remove from hot plate and let cool to room temperature.
- Spin coat SU-8 2002 onto the surface of the wafer for 40 sec at 500 rpm.
- Spin coat SU-8 2050 onto the wafer with a two-step spin coater program. Step 1: 40 sec at 500 rpm. Step 2: 1 min at 1,500 rpm.
- Remove the wafer from the spin coater and place on a preheated hotplate (65 °C) for 10 min.
- Let cool to room temperature, then place mask over wafer.
- Place under ultraviolet lamp and expose for 30 sec at 200 watts.
- Remove mask and place the wafer on a preheated hotplate (95 °C) for 10 min.
- Place in SU-8 Developer solution and lightly agitate until all unexposed SU-8 has been removed. Then rinse in a stream of isopropyl alcohol for 30 sec, blow dry with nitrogen.
- Place on a preheated hotplate (95 °C) for 30 min for a final hardbake.
- Die casting of PDMS pillars
- Mix vigorously a 10:1 mass ratio of PDMS sylgard-184 base to curing agent in beaker.
- Degas PDMS in a vacuum chamber until all bubbles are gone.
- Place the mold fabricated in section 1.1 in a large 4 in plastic weighing dish and pour the PDMS.
- Place dish with PDMS and mold back into vacuum chamber. Degas again until all bubbles are gone.
- Place entire dish in an oven (preheated to 75 °C) for at least 2 hr. Then let cool to room temperature.
- Cut away the dish from the PDMS, and the PDMS from the silicon wafer with a straight razor blade.
- Cut out PDMS region with the pillars from the bulk and store in a clean Petri dish.
2. Functionalization of the Tops of the Pillars
This three-step process involves first the evaporation of a gold film on a silicon wafer, followed by imprint transfer lithography16 of the gold film onto the PDMS pillars (fabricated in section 1), and lastly the functionalization of the gold film with a self-assembled monolayer to render it hydrophilic.
- Fabrication of gold on silicon wafers for imprint transfer lithography
- Use a glass cutter to dice a 4 in circular silicon wafer into 4 equally sized pieces. Note: Wafers can be cleaned using steps 1.1.2-1.1.4 and reused.
- Evaporate 20 nm of gold directly onto the silicon wafer.
- Leave the wafer in evaporation chamber (or in a desiccator) until section 3 below is complete. This will keep the wafer as clean as possible.
- Prepare an 8 µl:20 ml, (3-mercaptopropyl)-trimethoxysilane (MPTS) : toluene solution in a clean glass vial.
- Prepare 200 ml of 16 mM hydrochloric acid (HCl) in a clean beaker.
- Put the wafer with gold film into the plasma reactor.
- Clean the wafer using oxygen plasma at a pressure of 300 mTorr, power of 50 W for 10 min.
Note: For this procedure a home-built plasma reactor was used. - Put the wafer in a Pyrex Petri dish full of 200 proof ethanol for at least 10 min.
Note: This step is done to remove any unstable oxides that form on the gold due to the oxygen plasma. - Rinse the wafer with ethanol, then blow dry with nitrogen.
- Spin coat the MPTS solution onto the wafer at 500 rpm for 30 sec followed by 2,750 rpm for 1 min.
Note: MPTS is used as an adhesion layer between the PDMS and gold layer16. - Take the wafer off of the spin coater and rinse under a stream of ethanol. Then, rinse with DI water and blow dry with nitrogen.
Note: Rinse gently to avoid peeling of the gold layer from the silicon wafer. - Place the wafer into a Pyrex Petri dish that contains enough 16 mM HCl solution to fully submerge the wafer. Leave in HCl for at least 5 min.
Note: Place into the solution gently to prevent the gold from peeling off.
Note: This is done to improve the adhesion between the PDMS and gold layer16. - Remove the wafer from the HCl solution and blow dry with nitrogen.
Note: wafers should be used no more than 15-20 min after this step is complete.
- Imprint transfer lithography of the gold from wafer to PDMS pillars
- Prepare one 25 mm x 75 mm glass slide for each PDMS sample by rinsing it with ethanol, DI water, and blow dry with nitrogen.
- Place PDMS pillars into plasma chamber and perform oxygen plasma at a pressure of 300 mTorr and power of 50 W for 30 sec.
Note: overexposure of the PDMS to the oxygen plasma will cause cracking. Adjust the plasma conditions accordingly. - Bind the back of the PDMS substrates to the clean glass slides by applying light pressure to them. The glass slide facilitates the manipulations of the PDMS pillars and mounting on the device described in step 3.
- Flip the glass-backed PDMS substrates and press the pillars down onto the MPTS-functionalized gold films (step 2.1). Apply moderate pressure initially, and then put a weight (approximately 100 g) on the glass slide to ensure conformal contact.
- Leave the substrate in contact with the silicon wafer for at least 12 hr.
- Separate the PDMS substrate from the wafer. If the PDMS substrate is stuck, use a straight razor blade to carefully pry an edge of the PDMS off of the wafer.
- At this point a uniform gold film should be present on the top of the PDMS pillars. Use an optical microscope to verify that the gold film is not cracked or that there are no parts missing along the pillar.
- Functionalization of the gold on the top of the PDMS pillars
- Prepare enough 1 mM mercaptohexadecanoic acid (MHA) in dimethyl sulfoxide (DMSO) to submerge fully the gold on top of the PDMS pillars.
Note: DMSO is used for its low PDMS swelling factor17. - Place the PDMS substrates in the MHA solution and keep them there for at least 24 hr.
- Remove the substrate from the MHA solution and rinse with DI water, then blow dry with nitrogen.
- Place in vacuum chamber (pressure < 100 mTorr at 25 °C) for at least 12 hr.
- Prepare enough 1 mM mercaptohexadecanoic acid (MHA) in dimethyl sulfoxide (DMSO) to submerge fully the gold on top of the PDMS pillars.
Note: To verify that the functionalization process was successful, step 2 can be performed on a bulk piece of PDMS (without pillars) and the wetting angle can be tested in a goniometer. The MHA gold films should have advancing and receding water contact angles of <15° and ~0°, respectively.18
3. Formation and Characterization of the Capillary Bridges
This section details how a liquid bridge can be introduced between two substrates followed by its characterization via imaging at different heights and fluid volumes.
- Using two pillar substrates (made in steps 1-2), place one in the top and one in the bottom holders. Secure the substrates using side tension screws.
Note: see Figure 1 and representative results for device details. - Assemble the device by attaching the top substrate stage to the bread board such that the top substrate is roughly above the bottom substrate. Decrease the height between the two facing pillars to about 1mm.
- Rough alignment: using the x, y, and rotation knobs on the bottom substrate stage align (by eye) the gold strips for the two substrates so that they are parallel (looking top down through the top substrate).
- Fine alignment: position the camera to look down the length of the PDMS pillar. Using the live camera feed on the computer screen, further adjust the position of the bottom substrate so the pillars are parallel.
- Move the camera to the opposite side of the device and repeat step 3.4.
- Decrease the separation between the two pillars until the top pillar makes contact with the bottom pillar (using live camera feed). Zero the digital micro stage. This will be defined as a pore height of zero.
- Increase the pore height to approximately 200 µm.
- Prepare a syringe with 1-5 µl of an 80% glycerol, 20% water solution. Attach a 30 G needle to the end of the syringe, making sure no air bubbles get trapped inside the needle.
Note: the water/glycerol mixture is used to reduce evaporation during the experiment. Water can also be employed. - Mount the syringe to the syringe xyz translation stage with a mechanical clamp.
- Adjust the micrometers on the syringe positioning stage so that the needle fits into the slit pore (parallel to the length of the pillars).
- Decrease the slit pore height so that the top and bottom surfaces gently contact the needle. This will make sure that the liquid will touch both surfaces and spontaneously form a capillary bridge.
- Dispense the liquid from the syringe into the slit pore slowly.
- Use the micrometers on the syringe positioning stage to remove the needle from the slit pore.
Note: At this point, the height of the slit pore can be varied and the liquid bridge imaged.
Note: The pictures can be analyzed with the open source software package ImageJ.
Representative Results
Description of the experimental device
The experimental device can be broken up into four main parts: 1) the top substrate stage, 2) the bottom substrate stage, 3) the syringe/ syringe xyz-translation stage and 4) the camera/optics and camera holder. The details of each follow:
- Top substrate stage. A digital translation stage is attached to a P-series mounting clamp via a custom machined connector piece. The mounting clamp is connected to a variable height P-post, which is anchored to a bread board via a P-series clamping fork. A custom connection piece attaches to a custom machined glass slide holder to the translation stage, providing 1 µm displacement resolution in the z-direction.
- Bottom substrate stage. A xy linear translation with θ-axis rotation stage is attached to the bread board via 8 post extension pieces. A custom machined substrate holder is attached to the top of the xy linear translation with θ-axis rotation stage, allowing the bottom substrate to be positioned with 10 µm translational resolution and rotated with 1° resolution.
- Syringe/syringe xyz translation stage. For xyz positioning of the syringe used to fill the gap between the pillars, a 5 μl syringe with a 30 G needle is attached to a xy translation stage. The xy stage is then attached to a z translation stage via a 90° connector piece.
- Camera/ optics and camera holder. For imaging of the liquid bridges, a CCD camera is attached to a variable zoom optics piece. At maximum zoom, this gives a resolution of 3.3 µm/pixel. The camera is attached to a laboratory scissor jack, which can be positioned to image the liquid bridge from different angles.
Transfer of Au foil to PDMS pillars
In the transfer of the gold to the PDMS substrate, it is important to separate the PDMS device from the silicon wafer smoothly and carefully (see step 2.2.6). Figure 3a shows a microscope image of a PDMS pillar with gold after a successful transfer. Figure 3b shows excess gold foil from the wafer that was transferred to the pillar due to poor transfer. To facilitate the transfer of the gold film a sharp safety razor can be used to gently pry one edge of the PDMS pillar from the silicon wafer. Additionally, the PDMS substrate should be pulled in a direction normal to the wafer's surface (avoid lateral motion) to prevent additional gold foil from sticking to the edge of the substrate. Figure 3c shows how cracks can form in the gold layer after transfer if the PDMS substrate undergoes significant shear or bending.
Characterization of the MHA monolayer
Once the fabrication process (step 2) is finished, it is important to verify the quality of the MHA monolayer by testing its water contact angle. Figure 2 shows a liquid water drop on an Au/PDMS substrate after being functionalized with MHA. The low contact angle on the PDMS indicates that the process was successful. The inset of Figure 2 shows a liquid water drop placed on one of the raised pillars after the completed procedure. The 140° contact angle demonstrates that the combination of physical and chemical heterogeneities allow the drop to be pinned on the sides of the pillars.
Visualization of capillary bridges
Once the substrates have been fabricated and installed into the microstage holders, the channels can be filled using the syringe/ syringe xyz translation stage. Figure 4a shows a filled slit pore with a perspective perpendicular to the width of the pillar (looking "down the barrel" of the channel). Figure 4b shows a perspective orthogonal to Figure 4a, that is, perpendicular to the length of the slit pore. Figure 4c shows the process of filling the channel from the same perspective as Figure 4b. It is critical during the filling stage to dispense the liquid from the syringe slowly. The force from sudden large flow rates can depin the liquid from the top of the pillar, causing it to spread onto the hydrophobic PDMS regions. If this happens, the substrates must be cleaned and dried and the filling process repeated.
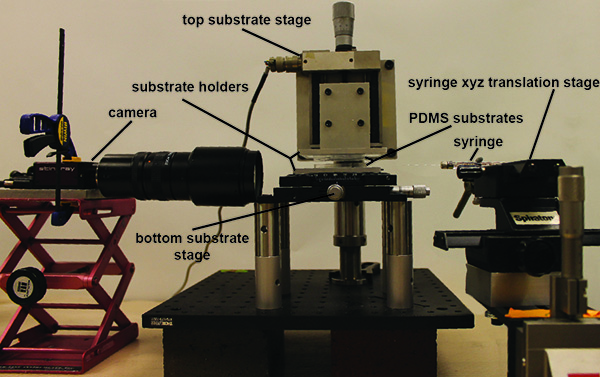
Figure 1. Picture of complete experimental setup. The PDMS substrates are held at a variable distance apart though a combination of x,y,z and rotation stages. A separate set of microstages (far right) holds the syringe to introduce the liquid into a narrow gap to create the capillary bridge in a slit-pore geometry. A CCD camera (pictured left) is used to image the resulting capillary bridges as the pore separation is changed. The resulting images can then be analyzed in the open source image analysis software ImageJ. Click here to view larger image.
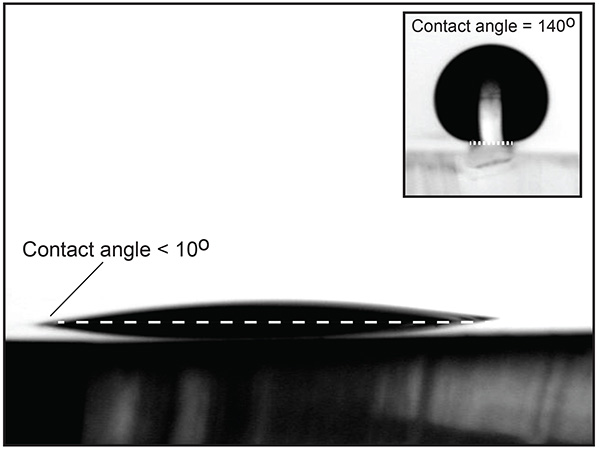
Figure 2. PDMS substrate with 20 nm Au layer functionalized by a MHA self-assembled monolayer. The low water contact angle shows that the procedure was successful. The inset shows a drop on a raised functionalized PDMS/Au pillar. Click here to view larger image.
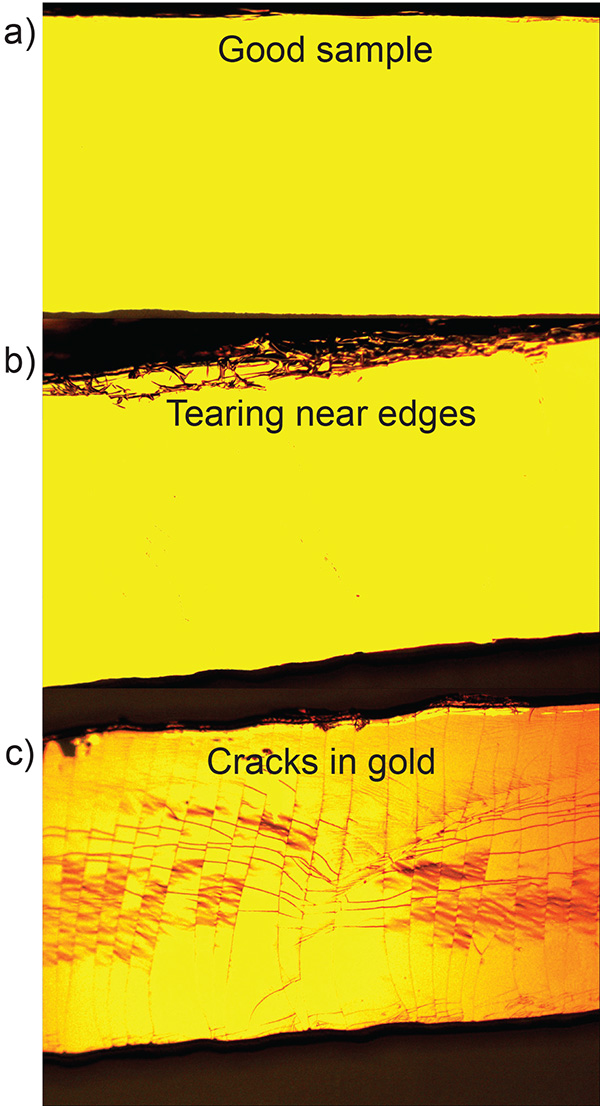
Figure 3. Raised PDMS pillar after transfer of 20 nm Au layer. a) Successful transfer. b) Tearing due to lateral motion of the PDMS substrate during the transfer process. c) Cracking caused by the bending of the PDMS substrate during the transfer process. Click here to view larger image.
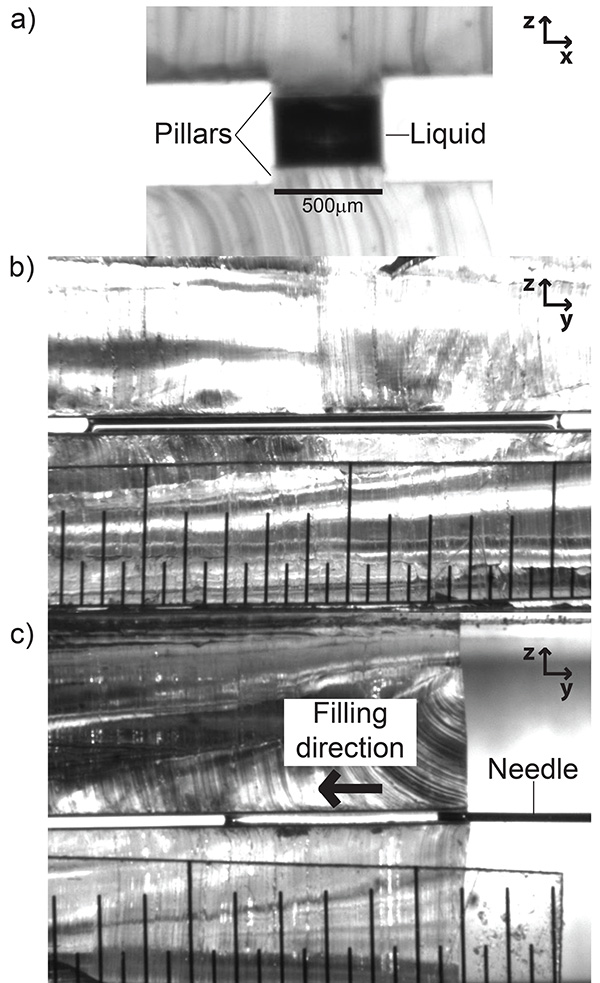
Figure 4. Images of capillary bridges on pillars in the experimental device. a) Field of view parallel to the length of the pillar. b) Field of view perpendicular to the length of the pillar. c) Shows the filling process of the slit pore (same perspective as b). The minor graduation of the ruler in b) and c) is 500 µm. Click here to view larger image.
Discussion
The method presented here provides a way to create capillary bridges in slit pore geometry, and also a method for imaging these bridges so that their morphology can be analyzed and compared to simulation and theory.
This method incorporates physical relief as well as selective chemical patterning to create asymmetric wetting properties. If only a chemical heterogeneity is present, a liquid drop will stay pinned on the heterogeneity until the contact angle exceeds that of the less wettable (lower surface energy) region. When PDMS is the region of lower surface energy, the maximum achievable contact angles at the hydrophilic/hydrophobic boundary is around 100°. Adding a physical heterogeneity in the form of a pillar allows for significantly larger water contact angles at the edge of the pillars (>140°), as seen in Figure 2 (An alternate method for creating similar substrates is presented by Ferraro et al.19). Higher contact angles imply that liquid drops or bridges can be confined to specific areas and sustain higher pressures than would be possible for a purely chemical heterogeneity.
Since the gold on top of the PDMS pillars is functionalized with a self-assembled monolayer, different functionalizations are possible using different thiol precursors. Also, in addition to being able to adjust the height of the slit pore, the combination of microstages allows for real time adjustment of both lateral and rotational offsets. This functionality would make such a device ideal for imaging dynamic capillary bridges systems, such as those relevant to ink jet or gravure printing.
Critical steps within the protocol
To obtain reproducible capillary bridge morphologies precautions must be taken in the preparation of the chemical and physical heterogeneities. For example, pillars with a thickness gradient lead to bridges located at the end of the pore where the PDMS is thicker. Thickness gradients can arise if the plastic weighing dish holding the liquid PDMS during the molding step is not lying perfectly flat. The change in height along the length of the pore can also lead to a change in the curvature of the liquid, skewing image data. The extent of this thickness variation can be evaluated when the zero point is set in step 3.6. The tilt of the top substrate holder can be reduced by placing a soft spacer between the top substrate holder and the substrate holder-z stage connector piece (a few layers of masking or foam tape works well for this). By varying the tension on the screws that attach the connector piece to the substrate holder, the tilt can be eliminated from the system.
It is also important to ensure that no excess DMSO is left on the substrates after the 24 hr DMSO/MHA soak. It is possible that a small amount of residual DMSO can be present on the substrate even after rigorous rinsing with DI water. If the substrates are used at this point, the excess DMSO can leach into the capillary bridge. Excess DMSO can be evaporated from the sample by placing it in a vacuum chamber (pressure <100 mTorr, 25 °C) for at least 12 hr.
Limitations of the technique
A chief limitation of using raised pillars to form high aspect ratio capillary bridges becomes apparent during imaging. When the height of the pore is changed at constant volume, the liquid recedes away from the ends towards the center of the pore1. As a consequence, the bridge can become out of focus when imaging normal to the width of the strip. This loss in focus happens when the distance between the end of the slit pore and the liquid bridge exceeds the depth of field of the camera. It is therefore important to use the shortest possible slit pore lengths required for a given experiment. The depth of field can be extended by changing optics, or by decreasing the magnification, but these come at a cost to resolution.
The tops of the PDMS pillars are functionalized to have a high surface energy (low water contact angle). As a consequence they are susceptible to contamination, coming either from the ambient environment or the fluid. Our experiments were performed in a cleanroom (class 1000) which allowed us to test the samples 5-10 times before any degradation of the surface was noticeable. Contamination leads to pinning of the wetting angle for the contact line parallel to the width of the pillars.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors are grateful for the support of the National Science Foundation under Grant No. CMMI-00748094 and the ONR N000141110629.
Materials
| 99.999% Gold wire | Kurt J. Lesker | EVMAU40040 | |
| Acetone | Pharmco-AAPER | C1107283 | |
| Dimethyl sulfoxide | Fisher | D128-500 | |
| Ethanol (200 proof) | Pharmco-AAPER | 111000200 | |
| Hydrochloric acid | EMD | HX0603-4 | |
| Hydrogen peroxide (30%) | EMD | HX0635-3 | |
| Isopropyl alcohol | Fisher | L-13597 | |
| Mercapto hexadecanoic acid (90%) | Sigma-Aldrich | 448303-1G | |
| Mercapto-propyl-trimethoxy-silane (MPTS) | Gelest | Sim6476-O-100GM | |
| Milli-Q DI water | Millipore | Milli-Q | |
| Nitrogen (gas) | Airgas | UN1066 | |
| Oxygen (gas) | Airgas | UN1072 | |
| Silicon wafers (4 in) | WRS Materials | CC8506 | |
| SU-8 2002 (negative photo resist) | MicroChem | SU82002 | |
| SU-8 2050 (negative photoresist) | MicroChem | SU82050 | |
| SU-8 Developer solution | MicroChem | Y020100 4000L1PE | |
| Sulfuric acid | J.T. Baker | 9681-03 | |
| Poly dimethy sulfoxide (PDMS) | Dow Corning | Sylgard -184 | |
| Toluene | Omnisolv | TX0737-1 |
References
- Broesch, D. J., Frechette, J. From Concave to Convex: Capillary Bridges in Slit Pore Geometry. Langmuir. 28, 15548-15554 (2012).
- Orr, F. M., Scriven, L. E., Rivas, A. P. Pendular rings between solids – meniscus properties and capillary force. J. Fluid Mech. 67, 723-742 (1975).
- Rose, W. Volumes and surface areas of pendular rings. J. Appl. Phys. 29, 687-691 (1958).
- Erle, M. A., Dyson, D. C., Morrow, N. R. Liquid bridges between cylinders, in a torus, and between spheres. Aiche J. 17, 115-121 (1971).
- Lambert, P., Chau, A., Delchambre, A., Regnier, S. Comparison between two capillary forces models. Langmuir. 24, 3157-3163 (2008).
- Mason, G., Clark, W. C. . Liquid Bridges Between Spheres. Chem. Eng. Sci. 20, 859-866 (1965).
- De Souza, E. J., Brinkmann, M., Mohrdieck, C., Arzt, E. Enhancement of capillary forces by multiple liquid bridges. Langmuir. 24, 8813-8820 (2008).
- Hornbaker, D. J., Albert, R., Albert, I., Barabasi, A. L., Schiffer, P. What keeps sandcastles standing. Nature. 387, 765-765 (1997).
- Scheel, M., et al. Morphological clues to wet granular pile stability. Nat. Mater. 7, 189-193 (2008).
- Mastrangeli, M., Ruythooren, W., Celis, J. -. P., Van Hoof, C. Challenges for Capillary Self-Assembly of Microsystems. IEEE T. Compon. Pack. 1, 133-149 (2011).
- Josell, D., Wallace, W. E., Warren, J. A., Wheeler, D., Powell, A. C. Misaligned flip-chip solder joints: Prediction and experimental determination of force-displacement curves. J. Electron. Pack. 124, 227-233 (2002).
- Lin, W., Patra, S. K., Lee, Y. C. Design of Solder Joints for Self-Aligned Optoelectronic Assemblies. IEEE T. Compon. Pack. B. 18, 543-551 (1995).
- Berthier, J., et al. Capillary self-alignment of polygonal chips: a generalization for the shift-restoring force. Microfluid. Nanofluid. 14, 845-858 (2013).
- Lambert, P., Mastrangeli, M., Valsamis, J. B., Degrez, G. Spectral analysis and experimental study of lateral capillary dynamics for flip-chip applications. Microfluid. Nanofluid. 9, 797-807 (2010).
- Mastrangeli, M., Valsamis, J. B., Van Hoof, C., Celis, J. P., Lambert, P. Lateral capillary forces of cylindrical fluid menisci: a comprehensive quasi-static study. J. Micromech. Microeng. 20, 10-1088 (2010).
- Childs, W. R., Nuzzo, R. G. Large-area patterning of coinage-metal thin films using decal transfer lithography. Langmuir. 21, 195-202 (2005).
- Lee, J. N., Park, C., Whitesides, G. M. Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem. 75, 6544-6554 (2003).
- Olivier, G. K., Shin, D., Gilbert, J. B., Monzon, L. A. A., Frechette, J. . Supramolecular Ion-Pair Interactions To Control Monolayer Assembly. Langmuir. 25, 2159-2165 (2009).
- Ferraro, D., et al. Morphological Transitions of Droplets Wetting Rectangular Domains. Langmuir. 28, 13919-13923 (1021).

