An Inverse Analysis Approach to the Characterization of Chemical Transport in Paints
Summary
In this paper, a procedure for quantifying the mass transport parameters of chemicals in various materials is presented. This process involves employing an inverse-analysis based diffusion model to vapor emission profiles recorded by real-time, mass spectrometry in high vacuum.
Abstract
The ability to directly characterize chemical transport and interactions that occur within a material (i.e., subsurface dynamics) is a vital component in understanding contaminant mass transport and the ability to decontaminate materials. If a material is contaminated, over time, the transport of highly toxic chemicals (such as chemical warfare agent species) out of the material can result in vapor exposure or transfer to the skin, which can result in percutaneous exposure to personnel who interact with the material. Due to the high toxicity of chemical warfare agents, the release of trace chemical quantities is of significant concern. Mapping subsurface concentration distribution and transport characteristics of absorbed agents enables exposure hazards to be assessed in untested conditions. Furthermore, these tools can be used to characterize subsurface reaction dynamics to ultimately design improved decontaminants or decontamination procedures. To achieve this goal, an inverse analysis mass transport modeling approach was developed that utilizes time-resolved mass spectroscopy measurements of vapor emission from contaminated paint coatings as the input parameter for calculation of subsurface concentration profiles. Details are provided on sample preparation, including contaminant and material handling, the application of mass spectrometry for the measurement of emitted contaminant vapor, and the implementation of inverse analysis using a physics-based diffusion model to determine transport properties of live chemical warfare agents including distilled mustard (HD) and the nerve agent VX.
Introduction
The mass transport mechanisms associated with contamination of materials by chemical warfare agents are driven by a variety of convolved processes including physical state transitions, chemical interactions between mobile species, and materials interfaces. To develop efficacious decontamination technologies, optimized decontamination procedures, and predictive models, it is vital that the contamination process is well understood, including the transport of contaminants into materials via absorption and the subsequent chemical emission back into the environment. Consequently, it is imperative that approaches are developed that can evaluate subsurface concentration profiles for contaminant-material pairs as a function of environmental conditions. A continuum-scale, physics-based model was developed to predict the concentration distribution of absorbed agent in a contaminated substrate. Experimentally derived mass transport parameters enable the prediction of the vapor emission from the contaminated material post decontamination. An ability to predict the concentration distribution in a material can facilitate the assessment of potential vapor hazards and, in turn, enable accurate diagnoses of toxicological hazards1. This approach allows for an estimation of contaminant-material pair specific mass transport parameters such as diffusivity and saturation concentration that in turn permit modeling for a other scenarios and conditions. In this study, we have treated the liquid phase contamination of solvent-dispersed, polyurethane paint coatings with chemical warfare agents bis(2-chloroethyl) sulfide (distilled mustard, blister agent HD) and O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate (VX), an organophosphate nerve agent.
The developed methodology characterizes gas desorption profiles from contaminated materials, including chemical warfare agents like HD and VX, without many of the restrictions that hamper other approaches2,3. Time-resolved mass spectrometry measurements of contaminant evolution from contaminated substrates allow for a diffusive transport model with inverse analysis to calculate mass transport parameters for the contaminant in the material, including the absorbed concentration profile for the contaminant starting from the original permeation event. With the establishment of a predictive capability for delineating concentration profiles of contaminants in materials as a function of environmental conditions comes the ability to assess toxicological hazards and ultimately develop routes for efficacious decontamination.
In this paper, the details associated with sample preparation are presented, including work with chemical warfare agent contaminants, as well as experimental data collection from contaminated materials and subsequent modeling4. Experimental runs were conducted as described in the chemical contaminant and decontaminant source document5 and will be discussed in the next section. A flow chart for sample preparation and analysis steps in included in Figure 1.
Protocol
1. Condition Paint Substrates to the Desired Environment
- Preset the environmental chamber for substrate conditioning to the specified temperature and relative humidity (20 °C, 50%). Ensure that the substrate conditions are consistently maintained since both temperature and water content can significantly influence absorption rates into materials.
- Coat 0.32 cm thick, 5.08 cm radius stainless steel discs with a surface area of 20.25 cm2 with a layer of paint (MIL-DTL-53039, a solvent-dispersible (SD) aliphatic polyurethane coating system6) with a total coating thickness (primer and top coat) of approximately 100 µm. Place the substrates (with desired number of replicates) on stainless steel trays with the test surface to be exposed to a chemical agent facing upwards.
- Cover the substrates with Petri dishes. Place the trays containing the test substrates into the environmental chamber for at least 60 min but ideally O/N, if possible.
2. Contamination of Preconditioned Substrates
- Don personal protective equipment such as lab coat, safety glasses, and gloves.
- CAUTION: Obtain the chemical contaminants from cold storage and allow primary container vial to equilibrate to RT. The chemicals used in this protocol are Chemical Agent Standard Analytical Reference Material (CASARM, 98.0% purity) grade HD and CASARM (89.0% high purity) grade VX (both liquid phase). Obtain purity information from either nuclear magnetic resonance or gas chromatography/mass spectroscopy analyses and maintain on file.
NOTE: CAUTION: The handling of chemical warfare agents should only be performed by trained personnel at an approved facility using applicable safety, security, and surety precautions. - CAUTION: Fit the agent delivery tool with a pipette tip and configure the tool to deliver a droplet of agent (e.g., 1 µl). Uncap the contaminant vial, and place the cap on the hood surface, threads facing up. Pick up the pipette, and slowly lower the tip into the contaminant solution. Load the agent delivery tool with agent in accordance with the manufacturer’s directions. Gently place the loaded pipette onto the hood working surface, and recap the contaminant vial.
- CAUTION: Contaminate the test substrates.
- Remove the trays containing the test substrates from the environmental chamber.
- Remove the Petri dishes from the test substrates, and set on the hood surface. Digitally photograph each test substrate to record the appearance of each substrate before contamination.
- Pick up the loaded pipette and deliver a single droplet (e.g., 1 µl) of agent onto the first test substrate. Cover the contaminated material with a polystyrene Petri dish to minimize evaporation while contaminating additional substrates. Repeat for each substrate; reload the pipette tip (Step 2.3) as necessary.
NOTE: Collect pipette confirmation samples before and after dosing of substrates for analysis via chromatography to confirm contaminant mass delivery. - Remove the Petri dishes from the test substrates and set on the hood surface. Digitally photograph each test substrate to record the initial contaminant-material interactions.
- Cover the test substrates with the Petri dishes.
- Place the tray of substrates back into the environmental chamber.
3. Contaminant-material Interaction Aging Period
- Prior to aging the samples, vent the stainless steel high vacuum experimental chamber to prepare it for use in Section 4.
- Allow the contaminated substrates to age in the environmental chamber for the specified duration, which can be varied based on the chemical-material-environment interaction dynamics (e.g., 60 min).
- Remove substrates from the environmental chamber and place on the working surface of the hood. Remove the Petri dishes from the test substrates and digitally photograph each substrate to record the post-aging contaminant-material interactions.
- Double-contain the test samples (e.g., multiple airtight containers) and transfer to the working hood with high vacuum chamber.
4. High Vacuum Vapor Emission Chamber Measurement
The high vacuum chamber is a small volume vessel pumped by a turbo molecular drag pump and a diaphragm backing pump (Figure 2). A quadrupole mass spectrometer is mounted on a port that directly faces a temperature-controlled substrate holder and is used to measure real-time gas evolution from contaminated substrates under high vacuum conditions. Full details on the vacuum chamber specifications and materials are included in reference 4.
- Ensure that the chamber is properly vented.
- CAUTION: Unpackage the samples prepared in Section 3, and remove Petri dish from the test substrates. Place one test substrate into each temperature-controlled substrate holder using stainless steel tweezers.
- Seal the vacuum chamber and begin the pump down sequence. Perform this step such that the chamber is vacuum sealed at the specified age time (e.g., 60 min) in relation to when the substrate was contaminated.
- Begin recording selected mass fragment channels as a function of time (direct measure of mass flux) identified with specific mass per unit charge values (m/z). Measure specific background gas species in addition to the primary mass fragments from the molecules of interest (VX: m/z = 114; HD: m/z = 109) in real time at <0.25 Hz until the contaminant partial pressure drops below detection limits of the mass spectrometer (10−8 Pa).
- Collect the emission curves for the duration of the emission of contaminant from the substrate.
- Stop recording emission curves with the mass spectrometer once the contaminant mass flux has decreased to the chamber pressure baseline.
- Vent the high vacuum chamber to atmospheric pressure.
5. Post-treatment Evaluation for Total Remaining Contaminant
- Open the high vacuum vapor emission chamber (HVVEC) instrument and remove the substrate from the chamber using stainless steel tweezers.
- Place the substrate into a glass extraction jar and add 20 ml of extraction solvent to the jar (e.g., isopropyl alcohol: VX; chloroform: HD). Cap the jar and swirl the jar three times. Leave the substrate in the extraction solvent for 60 min.
- Swirl the jar three times again and then uncap the jar. Using a clean, disposable, glass pipette, transfer approximately 1 to 2 ml of extraction solvent into an analytical vial for analysis via gas or liquid chromatography7 to measure the contaminant mass retained by the substrates.
6. Data Analysis and Modeling
- Convert raw mass spectrometry data (partial pressure derived from measured ion current) to mass flux from the substrate. Use a combination of the Hertz-Knudsen formula for converting partial pressure of detected gas species to incident vapor flux at the detector and include a scaling factor that accounts for the contaminated area on the substrate from the original contamination event.
- Use inverse methods (e.g., Levenberg-Marquardt algorithm) to determine the saturation concentration and diffusion constant values (key mass transport parameters) for the contaminant transport through the paint coating. Compare the experimentally determined vapor flux to a predicted vapor flux (an analytical solution to Fick’s second law with appropriate boundary conditions applied (see equation 4 from reference 4)).
- Use the mass transport parameters to predict the concentration profiles for the contaminant-material system.
Representative Results
The top panel of Figure 3 displays examples of the calculated mass flux of VX and HD from SD-painted substrates based on time-resolved mass spectrometry for the main mass fragments of VX and HD (mass-to-charge ratio, m/z = 114 and 109, respectively). A quadrupole mass spectrometer has three main components: an ionizer, a mass analyzer or filter, and a charge detector. Gas species are ionized via electron impact ionization (hot filament style electron source), and the produced ions are injected into a quadrupolar mass filter en route to reaching a Faraday cup or electron multiplier detector for measuring ion current (proportional to partial pressure of measured gases). For instrument used in this method, the ionizer was located in close (<3.5 cm), line-of-sight proximity to the substrate surface so that species emitted from a contaminated substrate (emission angle peaked at substrate normal) were detected preferentially and pseudo-instantaneously. The ions that comprise the ion current at the detector are assigned to mass fragments (m) with a specific ionization state (z) that results from the electron impact ionization process through the use of independent mass spectrometry standards and databases (e.g., NIST mass spectra database). The relative abundance of particular ions created in an electron impact ionization event is molecule-specific, and great care is needed in assigning mass spectral signatures especially for larger molecules, such as VX and HD, which rarely undergo single ionization or fragmentation events. Before time-resolved experiments were performed, full mass spectra for equivalent substrate preparation conditions were recorded to verify the presence and relative abundance of specific m/z peaks for the species introduced into the analysis chamber. This verification included confirming that the intensity of the expected main mass fragment for the analyte of interest was not convolved with contributions from other species in the system. The time-resolved measurements in subsequent experiments then recorded the intensity of specific m/z peaks as a function of time associated with the analytes of interest.
Raw mass spectrometry data are in the form of partial pressures of gases as measured at the ionizer of the spectrometer. For this study, the partial pressure values for the primary HD and VX fragments used for the flux calculations are based on a nitrogen ionization cross section standard with no correction for possible molecule-specific fragmentation efficiency. As specified in the protocol, Step 6.1, these partial pressure measurements at the detector are first converted to a mass flux using the Hertz-Knudsen formula that relates:
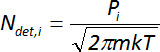 Equation 1
Equation 1
where Ndet,i, is incident molecular flux, Pi, is measured partial pressure (Pa), m is the molecular mass of the species (g/molecule), k is the Boltzmann constant (J/K), and T is the chamber temperature (K). Then, in order to determine the flux from the contaminated surface, Nc, the mass flux measured at the detector, Ndet, i, is multiplied by the ratio of the mass spectrometer detector area (cross sectional area of the detector parallel to the contaminated surface) to the area of contamination on the surface:
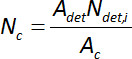 Equation 2
Equation 2
where Adet is the area of detector and Ac is the contaminated area on the substrate. The data shown in the top panel of Figure 3 focus on the time regime associated with diffusive mass transport for species originating from the subsurface of the substrate. Measurements prior to mass spectrometry demonstrated that both VX and HD drops did not remain sessile on the SD-paint with residual bulk liquid on the substrate prior to introduction to vacuum; the average final contaminated area for VX and HD was 2.6 ± 0.1 and 5.9 ± 0.6 cm2, respectively. Early time regime (<1,000 sec) data are not included in these plots, which are partly influenced by mass spectrometer equilibration for ionizer operation at relatively high partial pressures as well as desorption of bulk liquid contaminant that is trapped or weakly bound at the surface of the substrate. The data demonstrate a diffusion-limited regime that begins with an exponential decrease in mass flux rate and is the source of the data used for parameter estimation of diffusive transport from the subsurface of the contaminated substrate. The definition of the transition in regimes was confirmed by measuring evaporation of VX and HD liquid deposited on borosilicate glass substrates (impermeable material), which demonstrated a constant evaporation rate for both VX and HD until the deposited liquid was depleted. For the purposes of illustrating the various interactions that comprise the complex system under study, the bottom panel in Figure 3 shows the background signals measured from an uncontaminated paint substrate. Note the non-monotonic time evolution as well as the coincidence of features across species profiles. As measurements are recorded under vacuum conditions, any of the species trapped in the paint substrate are capable of diffusing out of the coating. This observation illustrates the possibility that the rate of mass flux out of the substrate (proportional to partial pressure) can be influenced by the relative abundance of rate of depletion of other species in the coating, including water and paint solvents.
Parameter estimation was performed using the diffusion-limited vapor emission flux. The calculated fit, which is an analytical solution to Fick’s second law of diffusion, allows for the determination of saturation and diffusivity constant for the specific contaminant-material combinations8. Vapor emission, including the mass flux magnitude as well as the time rate of change of the mass flux, from a material is driven by the contaminant distribution with respect to the material as well as the transport parameters associated with the contaminant in the material9-11. If the distribution of a contaminant is known, then the contaminant flux from the material can be predicted1. In contrast, if the vapor emission flux is measured (similar to the experimental procedure described herein), the concentration distribution in the substrate and transport parameters may be determined; a technique that is commonly referred to as inverse analysis12,13. In this experiment, an expression for vapor flux from the substrate can be derived via a one-dimensional (1D) analytical solution to Fick’s second law for the contaminant concentration profile in a finite thickness coating, assuming diffusive mass transport4,13. Figure 4 illustrates the different boundary conditions associated with constraining the analytical solution for molecular diffusion in a finite thickness absorbing coating. The full details of the calculation are documented elsewhere4, and the results are summarized in Table 1.
The mass transport parameters estimated from the vapor flux measurements were implemented to predict the contaminant concentration distribution and evolution within the SD paint coating. This result included representing the concentration field over the thickness of the paint at the start of diffusion of the contaminant out of the paint coating (after bulk liquid evaporation from the surface). The top panel of Figure 5 illustrates simulation results for the spatial-dependent concentration distribution of HD or VX in the SD paint coating. Although the coating absorbed a higher mass of HD, as indicated by a greater saturation concentration listed in Table 1, it is more localized near the surface of the substrate compared with VX. The bottom panel of Figure 5 illustrates the resulting vapor flux of the contaminants from the paints, which could yield health hazards to personnel in the vicinity of the material. The differences in transport phenomena between HD and VX demonstrate that intermolecular interactions can alter the transport of absorbing molecules through the paint systems to generate different distributions.
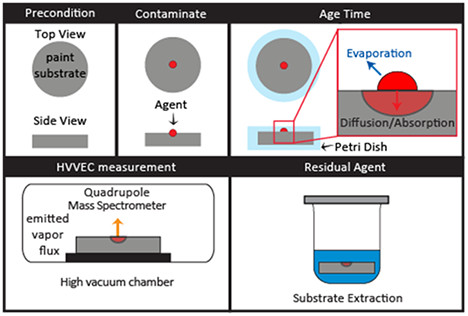
Figure 1. Substrate preparation and measurement flow chart that illustrates steps for preconditioning, contaminating, aging, and measuring subsequent emission of agent from substrates. Please click here to view a larger version of this figure.
Figure 2. Schematic of the high vacuum vapor emission chamber. Substrates are loaded onto a temperature-controlled substrate holder that directly faces a quadrupole mass spectrometer. The chamber has a base pressure (no substrate loaded) of <10−7 Pa and is pumped by a turbo molecular drag pump that is supported by a diaphragm backing pump. Substrates are introduced with the vacuum chamber vented, and measurements of mass flux at the mass spectrometer commence once the system is pumped down <10−2 Pa. Substrate diameter serves as a scale reference. Please click here to view a larger version of this figure.
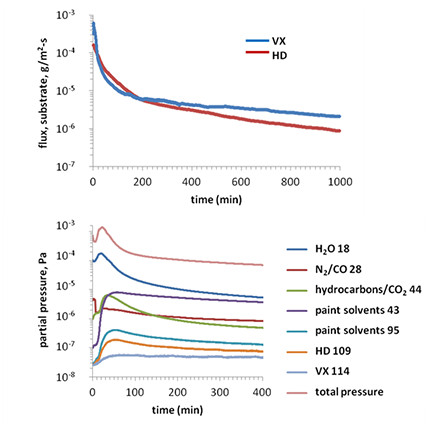
Figure 3. Top: VX (blue) and HD (red) calculated flux from substrates based on experimental measurement of vapor emission from contaminated SD chemical agent resistant coating substrates. The partial pressure (derived from ion current) measured at the mass spectrometer of the main fragments for a given analyte (VX: m/z = 114; HD: m/z = 109) can be related directly to the original mass flux from the substrate, assuming line-of-sight detection of species emitted from the material. Data shown are for the diffusion-limited mass transport regime only when bulk, surface-bound contaminant liquid has desorbed. Bottom: Background partial pressure measurements for gas species emitted by an uncontaminated paint substrate. For the purpose of illustrating the complexity of the system under study, the full time-resolved profiles are included from the initial point of collecting data (when total chamber pressure dropped below 10−2 Pa, the earliest point at which the mass spectrometer could record). The color key illustrates specific m/z values that correspond to the identified detected species.
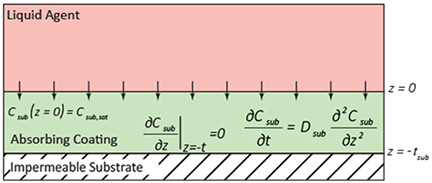
Figure 4. Definition of 1D molecular diffusive absorption governed by Fick’s law, including boundary conditions. Definition of variables: Csub, concentration of contaminant in the substrate; Csub,sat, saturation concentration; z, distance along the thickness of the absorbing coating; Dsub, diffusivity of contaminant in the coating; tsub, thickness of the paint coating. For the model that describes the measurement under vacuum for contaminant evolution from the substrate, the model assumes that at z = 0, there will be a vacuum-coating interface, the point at which diffusion-limited transport dominates. The form of the analytical solution to Fick’s law with these boundary conditions and its subsequent use with inverse analysis calculations are detailed in reference 4.
Figure 5. Top: Calculated concentration profiles from experimentally determined mass flux from paint substrates for each agent as a function of paint coating depth using inverse analysis. The profiles reflect the distribution at the point where the vapor flux from the contaminated material come solely from the subsurface and is determined by diffusive mass transport. Overall, in this case, more HD mass is absorbed and does so in a thinner portion of the paint film compared to VX. This difference is reflected in the calculated saturation concentration and diffusion coefficient values. Bottom: Calculated vapor flux profiles based on the starting point concentration profiles in the top portion of the figure. The calculated, time-dependent vapor flux matches the experimental data shown in Figure 3 with regard to predicting the relative difference in evolution of each agent. Please click here to view a larger version of this figure.
| Agent | Diffusivity (m2/s) | Saturation (g/m3) |
| VX | 9.91 ± 0.07 × 10–14 | 5.32 ± 0.03 × 104 |
| HD | 2.11 ± 0.04 × 10–14 | 1.17 ± 0.01 × 105 |
Table 1. Mass transport parameter estimation results for VX and HD interacting with SD paint. Table adapted from reference 4.
Discussion
Mass transport parameters for HD and VX in the paint were determined via the numerical inverse analysis of vapor emission data. With calculated parameters, it was possible to then produce time-dependent concentration gradient maps for contaminant distribution in the paint coating. The inverse analysis results demonstrated that the solubility of HD in the SD paint was higher than VX, but the diffusivity was approximately 5x lower. The results suggest that the HD contamination was highly concentrated at the surface of the coating, whereas the VX contamination profile penetrated through the SD paint coating. Therefore, decontamination requirements for SD paint will differ based on the chemical contaminant. Applications of this method provide a foundational understanding of contaminant absorption into materials and, ultimately, a predictive tool to characterize the contaminant resistance of materials.
Although the work presented here has shown promise, there are numerous factors to consider in systems like these that intrinsically contain many entangled interactions. To extend this approach to even more contaminant-material systems for different environmental conditions will require a continual evaluation of influences on mass flux measurements. These effects include desorption of multiple species from the bulk, other than the analytes of interest as alluded to in Figure 3, which may or may not alter the mass transport dynamics relative to other environmental conditions. For example, the vacuum measurement environment may cause a change in the absorbed composition and content (e.g., water and other organic solvents associated with paint) compared to ambient pressure conditions that can in turn alter the contaminant evolution profile.
Application of the technique to highly persistent chemical systems that require a larger dynamic range for partial pressure measurements may require an evaluation of instrument sensitivity with regards to the relationship between chamber pressure and measured ion current. Conversely, application of the technique to highly volatile or reactive chemical species may require careful consideration of the time between chamber evacuation and data collection (steps 4.3 and 4.4) to ensure viable mass flux data.
Finally, there may be other mechanisms14-16 that govern mass transport, such as porous transport and mutual diffusion or the need to account for the multilayer nature of paint coatings like primer and top coat layers. All of these possible influences with regards to characterizing contaminant transport are currently part of ongoing studies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. Wes Gordon (ECBC) for support in instrument design. This work represents the cumulative results from two research programs funded by Eric Lowenstein and Michael Roberts (Defense Threat Reduction Agency) under program CA08MSB317. The technical reports cited herein can be obtained at http://www.dtic.mil.
Materials
| Name of Material/Equipment | Company | Catalog Number | Comments/Description |
| Stainless Steel Tray | McMaster Carr | 4189T1 | 13-5/8" L x 9-3/4" W, http://www.mcmaster.com/#stainless-steel-trays/=p8dcgp |
| MIL-DTL-53039 solvent-dispersible aliphatic polyurethane coating system | Substrates supplied by internal source | ||
| Environmental Chamber | Custom Design. Full details on vacuum chamber specifications and materials included in reference 4. | ||
| bis(2-chloroethyl) sulfide | CASARM | TOXIC | |
| O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate | CASARM | TOXIC | |
| Pipetter | Fisher Scientific | 22260201 | Range of 1.0 µL to 10 mL |
| Pipetter Tips | Fisher Scientific | 13-683-709 | 0.1 mL Volume |
| Stainless Steel High Vacuum Experimental Chamber | Custom Design | ||
| Quadrupole Mass Spectrometer | ExTorr | RGA300 | |
| Stainless Steel Tweezers | McMaster Carr | 5516A15 | Any stainless steel tweezers are appropriate. |
| Glass Extraction Jar | Scientific Specialties | 170808 | Jar fits a ~5 cm diameter substrate. Different glass jars with teflon lined lids are appropriate for different sized substrates. |
| Chloroform | Sigma-Aldrich | 650498 | HARMFUL. The extraction solvent for HD may change depending on the analytical method. |
| Isopropanol | Sigma-Aldrich | 650447 | HARMFUL. The extraction solvent for VX may change depending on the analytical method. |
| Pasteur Pipette | VWR | 14673-010 | size= 5 3/4" |
References
- Willis, M. P., Mantooth, B. A., Lalain, T. Novel Methodology for the Estimation of Chemical Warfare Agent Mass Transport Dynamics, Part II: Absorption. J. Phys. Chem. C. 116, 546-554 (2011).
- Felder, R. M. Estimation of Gas Transport-Coefficients from Differential Permeation, Integral Permeation, and Sorption Rate Data. J. Membr. Sci. 3, 15-27 (1978).
- Taviera, P., Mendes, A., Costa, C. On the Determination of Diffusivity and Sorption Coefficients Using Different Time-lag Models. J. Membr. Sci. 221, 123-133 (2003).
- Willis, M. P., Gordon, W. O., Lalain, T. A., Mantooth, B. A. Characterization of Chemical Agent Transport in Paints. J. Hazard Mater. 260, 907-913 (2013).
- Lalain, T., Mantooth, B., Shue, M., Pusey, S., Wylie, D. . The Chemical Contaminant and Decontaminant Test Methodology Source Document. Second Edition. Report No. ECBC-TR-980. , (2011).
- . . MIL-DTL-53039B: Coating Aliphatic Polyurethane, Single Component, Chemical Agent Resistant. , (2005).
- Shue, M., et al. . Low-Level Analytical Methodology Updates to Support Decontaminant Performance Evaluations. Report No. ECBC-TR-883. , (2011).
- Schwope, A. D., Klein, J. M., Sidman, K. R., Reid, R. C. Sorption-Desorption Phenomena of Chemicals from Polymer (Paint) Films. J. Hazard. Mater. 13, 353-367 (1986).
- Li, F., Niu, J. Control of Volatile Organic Compounds Indoors – Development of an Integrated Mass-Transfer-Based Model and Its Application. Atmos. Environ. 41, 2344-2354 (2007).
- Li, F., Niu, J., Zhang, L. A Physically-Based Model for Prediction of VOCs Emissions from Paint Applied to an Absorptive Substrate. Build. Environ. 41, 1317-1325 (2006).
- Li, F., Niu, J. L. Simultaneous Estimation of VOCs Diffusion and Partition Coefficients in Building Materials via Inverse Analysis. Build. Environ. 40, 1366-1374 (2005).
- Li, F., Niu, J. L. An Inverse Technique to Determine Volatile Organic Compounds Diffusion and Partition Coefficients in Dry Building Material. Heat and Mass Transfer. 41, 834-842 (2005).
- Li, F., Niu, J. L. An Inverse Approach for Estimating the Initial Distribution of Volatile Organic Compounds in Dry Building Material. Atmos. Environ. 39, 1447-1455 (2005).
- Vesely, D. Diffusion of Liquids in Polymers. Int. Mater. Rev. 53, 299-315 (2008).
- Goossens, E. L. J., van der Zanden, A. J. J., Wijen, H. L. M., van der Spoel, W. H. The Measurement of the Diffusion Coefficient of Water in Paints and Polymers from Their Swelling by Using an Interferometric Technique. Prog. Org. Coat. 48, 112-117 (2003).
- Arya, R. K., Vinjamur, M. Measurement of Concentration Profiles Using Confocal Raman Spectroscopy in Multicomponent Polymeric Coatings-Model Validation. J. Appl. Polym. Sci. 128, 3906-3918 (2013).

