Taking Advantage of Reduced Droplet-surface Interaction to Optimize Transport of Bioanalytes in Digital Microfluidics
Summary
The protocol for fabrication and operation of field dewetting devices (Field-DW) is described, as well as the preliminary studies of the effects of electric fields on droplet contents.
Abstract
Digital microfluidics (DMF), a technique for manipulation of droplets, is a promising alternative for the development of “lab-on-a-chip” platforms. Often, droplet motion relies on the wetting of a surface, directly associated with the application of an electric field; surface interactions, however, make motion dependent on droplet contents, limiting the breadth of applications of the technique.
Some alternatives have been presented to minimize this dependence. However, they rely on the addition of extra chemical species to the droplet or its surroundings, which could potentially interact with droplet moieties. Addressing this challenge, our group recently developed Field-DW devices to allow the transport of cells and proteins in DMF, without extra additives.
Here, the protocol for device fabrication and operation is provided, including the electronic interface for motion control. We also continue the studies with the devices, showing that multicellular, relatively large, model organisms can also be transported, arguably unaffected by the electric fields required for device operation.
Introduction
The miniaturization of devices that work with liquids is of paramount importance for the development of “lab-on-a-chip” platforms. In this direction, the last two decades have witnessed a significant progress in the field of microfluidics, with a variety of applications.1-5 Contrasting with the transport of fluid in enclosed channels (channel microfluidics), DMF manipulates droplets on arrays of electrodes. One of the most attractive merits of this technique is the absence of movable parts to transport fluids, and motion is instantly stopped by turning off electric signals.
However, droplet motion is dependent on droplet contents, certainly an undesirable characteristic for a universal “lab-on-a-chip” platform. Droplets containing proteins and other analytes stick to device surfaces, becoming unmovable. Arguably, this has been the major limitation for broadening the scope of DMF applications;6-8 alternatives to minimize the unwanted surface fouling involve the addition of extra chemical species to the droplet or its surroundings, which could potentially affect droplet content.
Previously, our group developed a device to allow the transport of cells and proteins in DMF, without extra additives (Field-DW devices).9 This was achieved by combining a surface based on candle soot,10 with a device geometry that favors droplet rolling and leads to an upward force on the droplet, further decreasing droplet-surface interaction. In this approach, droplet motion is not associated with surface wetting.11
The goal of the detailed method described below is to produce a DMF device capable of transporting droplets containing proteins, cells, and entire organisms, without extra additives. The Field-DW devices pave the way for fully controlled platforms working largely independently of droplet chemistry.
Here, we also present simulations showing that, despite the high voltage required for device operation, the voltage drop across the droplet is a small fraction of the applied voltage, indicating negligible effects on bioanalytes inside the droplet. In fact, preliminary tests with Caenorhabditis elegans (C. elegans), a nematode used for a variety of studies in biology, show that worms swim undisturbed as voltages are applied.
Protocol
NOTE: In the procedures described below, laboratory safety guidelines must always be followed. Of particular importance is the safety when dealing with high voltage (> 500 V) and handling chemicals.
1. Coating of a Conductive Substrate with Candle Soot
- Cut copper metal into rectangles (75 x 43 mm, 0.5 mm thick). Clean each copper substrate by immersion in copper etchant for about 30 sec, wash with tap water for about 20 sec, and dry with paper.
NOTE: If using Method 1 below, change the dimensions to 75 x 25 mm to fit into the machine. - Sweep a lit paraffin candle under the copper substrate for 30–45 sec, to obtain an approximately uniform soot coating (about 40 µm thick). Keep the substrate at ~1 cm inside the flame. Do not touch the fragile soot surface.
2. Protecting the Soot Layer with Coating
NOTE: The soot layer is very fragile, and must be coated for protection. Two simple alternatives (methods 1 and 2 below) are suggested here, but more robust protocols are currently under development.
- Method 1
- Load the sample into the metal evaporator or sputtering system. Following the operation procedures of the system, evacuate the chamber, and start controlled deposition of gold onto the soot layer (150–200 nm). Let the device cool down to room temperature.
- Dip-coat the metalized substrate in a 1-dodecanethiol solution (1% v/v, in 95% ethanol, ACS/USP grade), for 10 min inside a chemical hood. Then, holding the device at an angle close to 60°, gently wash the surface with several droplets of ethanol only. Let the devices dry, overnight.
- Method 2
- In a chemical hood, immediately after coating the substrate with soot and while the substrate is still warm from the candle flame, deposit some droplets of fluorinated liquid on one side of the substrate, and tilt the substrate to an angle close to 90°. Deposit more droplets, and let them roll over the entire soot surface.
NOTE: When the droplet falls on a spot, soot will be washed away from that area. Let the droplets of fluorinated liquid spread as much as possible. - Bake the substrate on a hot plate (160 °C for 15 min) inside a chemical hood.
- Let the substrate sit overnight at room temperature before usage. Store indefinitely.
- In a chemical hood, immediately after coating the substrate with soot and while the substrate is still warm from the candle flame, deposit some droplets of fluorinated liquid on one side of the substrate, and tilt the substrate to an angle close to 90°. Deposit more droplets, and let them roll over the entire soot surface.
3. Fabrication of Top Electrodes (Adapted from Abdelgawad et al.12)
- Draw the electrodes using graphic design software. Each electrode is 2 mm long, 0.3 mm wide, and the gap between electrodes is 0.3 mm. The gap between contacts (to snap into the connector, see below) is 2.3 mm (Figure 1).
- Trim a flexible copper laminate (35 µm thick) into the Monarch format (3.87 x 7.5 inches). Use other sizes if compatible with the printer. Load the laminate into the manual feed tray of a color printer.
- Make sure to use “rich black”, or “registration black”, when printing on the copper sheet (see Abdelgawad et al.12 for details) to allow a denser layer of black ink on the copper substrate, protecting the printed pattern during etching. Let the printed substrate dry completely, overnight.
- Inside a chemical hood, warm up (40 °C) a beaker with 50 ml of copper etchant. Dip the printed laminate in the beaker, and gently shake it in the solution for about 10 min. Etching time depends on the copper etchant solution. Every few minutes, check the corrosion and see if the pattern is intact.
- Carefully wash the laminate with water, and remove the coating with acetone and ethanol in the chemical hood. Wash once again, and gently dry the laminate with paper towel.
- Carefully attach the laminate with electrodes to a glass slide (75 x 25 mm, ~1 mm thick), using double sided tape. Avoid air pockets.
- Attach a film of perfluoroalkoxy PFA to the electrodes using tape. This serves to prevent accidental contact of the electrodes with the droplet, which damages top electrodes due to short-circuit.
4. The Electronic Interface (Circuit in Figure 2)
- Solder the relays and the capacitors C to a universal circuit board.
- Assemble the remainder of the 10 relay drivers on a solderless breadboard for electronic circuits.
- Wire each relay driver’s input to a channel in the control board.
- Carefully snap the top electrodes into a connector (Figure 3). Wire each relay driver’s output to a top electrode, as shown in the figure. Note that there is a grounded connector contact between a pair of wires from relays, to minimize electrical noise.
NOTE: The connector sits on an adjustable platform to control the distance (0.1–0.5 mm) between top and bottom (soot-coated) substrate. - Use a program to control timing for high voltage (HV) application (about 0.8 sec) to 4 electrodes at the same time, shifting 1 electrode in the direction of motion (i.e., for 0.8 sec, actuate 1234; then 2345, 3456, etc., 0.8 sec for each group, and then backwards, so droplet moves in the opposite direction as well).
5. Droplet Visualization and Handling
- To record droplet motion, use the visualization system, which is composed of a 24X – 96X magnification assembly combined with a CCD camera. Connect the video recorder to the camera using S-video.
- Pipette a 4 µl droplet containing C. elegans in media on the bottom of the soot-coated substrate.
- Bring the top electrodes to ~0.3 mm above the droplet. The droplet should be close to the middle, just below the fifth electrode, for easier operation.
- Turn on the electronic interface and high voltage (500 VRMS), and adjust the top electrode distance to the droplet until it starts moving. Do not let the top electrodes touch the droplet.
- Collect data by recording the number of successful droplet transfers on the device in response to electric pulses. A successful experiment is characterized by at least 700 droplet transfers, i.e., one transfer after each electric pulse.
- Collect data continuously, until the droplet does not move anymore in response to 5 to 10 pulses.
NOTE: When the surface starts to degrade, motion could be restored by bringing the top electrodes closer to the droplet.
Representative Results
Previously, we have used Field-DW devices to allow the motion of proteins in DMF. In particular, droplets with bovine serum albumin (BSA) could be moved at a concentration 2,000 times higher than previously reported by other authors (without additives). This was due to the reduced interaction between droplet and surface; Figure 4 shows a droplet containing fluorescently-tagged BSA (see Freire et al.9 for more information on the experiments). The first picture on the left shows the droplet sitting on the soot-coated surface; the middle one, the effect of electric field, which, in addition to produce droplet rolling, also applies an upward force on the droplet, further reducing the interaction with the surface. Note the shear contrast (right) to a common alternative used in DMF, which is a surface coated only with fluorinated liquid (without candle soot); the strong interaction with the surface, indicated by the lower contact angle, quite often hinders motion.
Here, we use the experimental set-up (Figure 3) to continue the tests with these devices, now transporting droplets containing larger organisms, the worm C. elegans, a nematode used in a variety of biological assays.
Droplets with worms were successfully actuated on soot-coated substrates. In particular, Movie 1 shows a droplet moving in response to each voltage pulse (~0.8 sec interval) (note that the liquid fraction, stuck to a place with no soot, is out of the droplet pathway). Inspection after the experiments revealed that no worms, debris, or liquid residues, were left on droplets pathways after the experiments, indicating reduced interaction between droplet and surface.
The electronic interface (Figure 2) allows automation and better control of motion, since simultaneous actuation of groups of electrodes (Figure 1) increases the upward force, further reducing interaction with the surface.
Different experiments have shown that worms swim undisturbed as the droplet moves (20 min total actuation time), indicating that the high voltage (~500 VRMS) required for device operation is not harmful to the biological species being transported. This is supported by simulations, which have shown that the voltage drop across the droplet is an insignificant fraction (10-6%) of the voltage required for operation (Figure 5, potential difference between a point at the top and bottom of the plane in the middle of droplet); in fact, in droplets containing Jurkat T-cells, previous studies done by other authors13 suggest that such minimal voltage drops do not affect cell viability, proliferation, and biochemistry. For further validation, however, we are currently in the process of designing experiments to evaluate long term effects of the voltage on C. elegans. For the simulations described here, a ~2 µl droplet was assumed to be of PBS (phosphate buffer saline), sitting on a 30 µm thick layer of soot. Top and bottom electrodes were modeled as copper, and the applied voltage equal to 500 VRMS (For details on the simulations, see Freire et al.9).
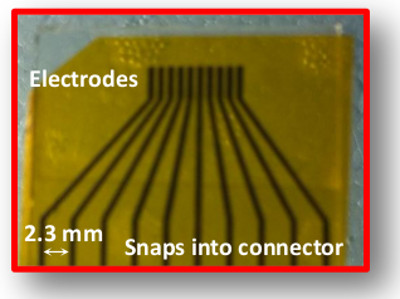
Figure 1: Picture of top electrodes. Each one of the 10 is 2 mm long and 0.3 mm wide. The gap between 2 electrodes is also 0.3 mm, and the gap between contacts (bottom) is 2.3 mm.
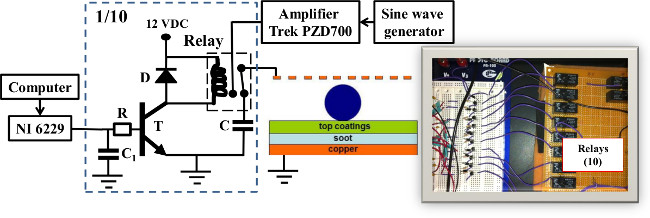
Figure 2: Schematic of the controlling system for top electrodes, detailing 1 of the 10 relay drivers. Each top electrode is either subjected to voltage, or connected to a capacitor. On the right, a picture of the board with the relays. Note that the high voltage required for operation is kept away from the control board (white base) on the left. Droplet diagram (Middle) adapted with permission from Freire et al.9 Please click here to view a larger version of this figure.
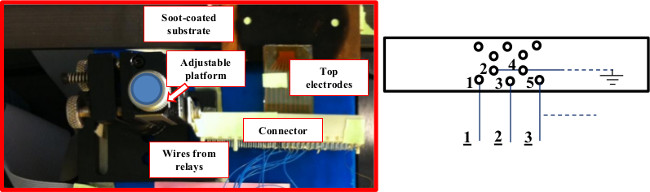
Figure 3: View of the experimental set-up. The distance between top and bottom (soot-coated) substrate is adjustable. The contacts from top electrodes are snapped into a connector. The wires from relays (shown here only 1, 2 and 3 of the 10 wires) are soldered to the connector, as indicated by the diagram on the right. Note that there is a grounded connector contact (e.g., connector contacts 2 or 4) between a pair of wires from relays (e.g., 1 or 3), to minimize electrical noise. Please click here to view a larger version of this figure.
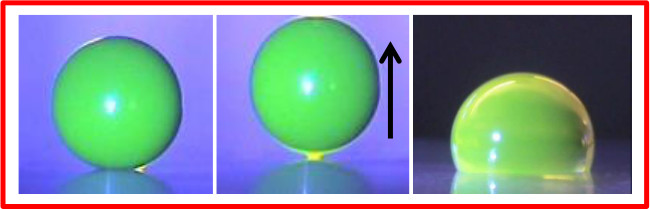
Figure 4: Droplets (4 µl) with Fluorescently-tagged BSA (10 g/L). Left, sitting on a soot-based substrate; middle, one of the effects of the electric field is to apply an upward force on the droplet, further minimizing the interaction with the substrate; right, droplet on a surface coated only with fluorinated liquid (no soot). Adapted with permission from Freire et al.9
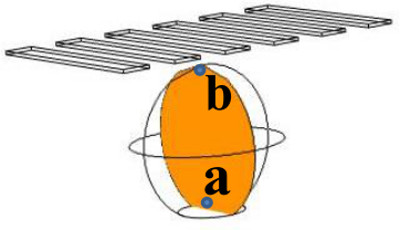
Figure 5: Simulations showing the voltage drop across a droplet in a Field-DW device. The potential difference between the top (point b) and bottom (point a) of the plane in the middle of the droplet is in the microvolt range, an insignificant fraction (10-6%) of the voltage for required operation.
Movie 1. Droplet with C. elegans on a Field-DW device, moving in response to each voltage pulse (~0.8 sec interval). The liquid fraction shown on the bottom left of the video is not in the droplet pathway.
Discussion
The most critical step of the protocol is the protection of the soot layer, directly associated with the success in moving droplets. Metallizing the soot layer (method 1 above) allows close to 100% of fabrication success. However, the maximum operation time is about 10 min; possibly, droplet fractions are wetting the soot through holes in the metal layer. Coating the soot layer with the fluorinated liquid is the easiest and fastest alternative, and requires minimum resources, but only 40–50% of the fabricated substrates work (20 min maximum) — and the coating is not uniform. In fact, the soot layer is very fragile, and the viscous fluorinated liquid easily damages it. We are currently working on more robust alternatives to protect the soot layer, which would increase the operation time of the device. However, one important aspect is the adsorption of droplet contents to the surface. Previously,9 we quantified the amount of protein that attached to the surface during device operation, and a correlation was found between continued motion and reduced surface adsorption of bovine serum albumin (BSA). Notwithstanding, biofouling is a complex matter, and some authors even suggest that it might be impossible to completely suppress the effect; in theory, if only a single protein attaches to a surface, more will be attracted to this site. In fact, the maximum operation time reported for digital microfluidic devices (by other authors6) was about 40 minutes. Therefore, the robustness of the surface is a point of great importance and still a work in progress.
Note that, in electrowetting, the application of voltage often spreads the droplet with analytes on the surface, entirely hindering motion, unless additives are used. However, some additives can be toxic, or might only work within a range of analyte concentration in the droplet. Field-DW devices allow the transport of analytes ranging from proteins to single cells and entire organisms, without extra additives. In addition, device characteristics are largely independent of thickness, uniformity, and electrical properties of the soot layer (see Freire et al.9 for more information).
Therefore, the significance of the method described here is that it broadens the scope of applications for DMF, paving the way for the development of fully controlled lab-on-a-chip platforms working largely independently of droplet chemistry.
The dimensions of top electrodes are compatible with the resolution of the printer, and are not unique; narrower and closer electrodes could also work. In fact, other methods for the fabrication of printed circuit boards in electronics can be used as well. What matters is that the droplet is subjected to a non-uniform electric field, and it will move towards the region where the field is more intense. However, care should be taken in the design to keep the electric field between energized and floating electrodes below 3 MV/m to prevent sparking; here, the field is about 1.7 MV/m, without sharp edges.
The operation of the electronic circuits is as follows. Each top electrode, through a relay contact, is either connected to the output of a high voltage amplifier, or to a capacitor (C), to minimize electrical noise. The transistor T allows the low current outsourced by the control board, through the resistor R and the capacitor C1, to control the larger current required for the relay coil to operate. The diode D prevents circuit damage due to the variable current in the coil (see materials list for list of components). Only one control board allows individual addressing of all electrodes, and only one HV power supply is required (Figure 2), which output voltages (8–18 kHz, 500–660 VRMS) after amplifying the sine wave provided by a generator. Note that HV is kept as far away as possible from the control system, to minimize noise and possible circuit malfunction.
The assays reported here used 4 µl droplets, simply due to the fact that smaller droplets containing C. elegans are more difficult to pipette. The culture of C. elegans will not be discussed here, and the reader should look for the protocols elsewhere (e.g., Brenner14).
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the Lindback Foundation for financial support, and Dr. Alexander Sidorenko and Elza Chu for fruitful discussions and technical assistance, and Professor Robert Smith for assistance with the C. elegans assays.
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description |
| Paraffin candle | Any paraffin candle | ||
| Sputtering system | Denton Vacuum, Moorestown, NJ | Sputter coater Desk V HP equipped with an Au target. | |
| 1-dodecanethiol | Sigma-Aldrich | 471364 | |
| Teflon | Dupont | AF-1600 | |
| Fluorinert FC-40 | Sigma-Aldrich | F9755 | Fluorinated liquid: Prepare Teflon-AF resin in Fluorinert FC-40, 1:100 (w/w), to create the hydrophobic coating. |
| Graphic design software -Adobe Illustrator | Adobe Systems | Other softwares might be used as well. | |
| Copper laminate | Dupont | LF9110 | |
| Laser Printer | Xerox | Phaser 6360 or similar | Check for the compatibility with "rich black" or "registration black" (see text). |
| Copper Etchant | Transene | CE-100 | |
| Perfluoroalkoxy (PFA) film | McMaster-Carr | 84955K22 | |
| Breadboard | Allied Electronics | 70012450 or similar | Large enough to allow the assemble of 10 drivers. |
| Universal circuit board | Allied Electronics | 70219535 or similar | |
| Connector | Allied Electronics | 5145154-8 or similar | |
| Control board and control program (LabView software) | National Instruments | NI-6229 or similar | |
| High-voltage amplifier | Trek | PZD700 | |
| Resistor R 27 kΩ, 1/4 W | Allied | 2964762 | |
| Capacitors C and C1, 100 nF, 60 V | Allied | 8817183 | |
| Transistor T, NPN | Allied | 9350289 | |
| Diode D, 1N4007 | Allied | 2660007 | |
| Relay | Allied | 8862527 | |
| Visualization system | Edmund Optics | VZM 200i or similar | System magnification 24X- 96X. It is combined with a Hitachi KP-D20B 1/2 in CCD Color Camera. |
| Recorder | Sony | GV-D1000 NTSC or similar | It is connected to the camera by an S-video cable. |
| Simulations | COMSOL Multiphysics | V. 4.4 |
References
- Fair, R. B. Digital microfluidics: is a true lab-on-a-chip possible. Microfluid Nanofluid. 3 (3), 245-281 (2007).
- Gupta, S., Alargova, R. G., Kilpatrick, P. K., Velev, O. D. On-Chip Dielectrophoretic Coassembly of Live Cells and Particles into Responsive Biomaterials. Langmuir. 26 (5), 3441-3452 (2009).
- Shih, S. C., et al. Dried blood spot analysis by digital microfluidics coupled to nanoelectrospray ionization mass spectrometry. Anal Chem. 84 (8), 3731-3738 (2012).
- Gorbatsova, J., Borissova, M., Kaljurand, M. Electrowetting-on-dielectric actuation of droplets with capillary electrophoretic zones for off-line mass spectrometric analysis. J Chromatogr. 1234, 9-15 (2012).
- Qin, J., Wheeler, A. R. Maze exploration and learning in C. elegans. Lab Chip. 7 (2), 186-192 (2007).
- Koc, Y., de Mello, A. J., McHale, G., Newton, M. I., Roach, P., Shirtcliffe, N. J. Nano-scale superhydrophobicity: suppression of protein adsorption and promotion of flow-induced detachment. Lab Chip. 8 (4), 582-586 (2008).
- Perry, G., Thomy, V., Das, M. R., Coffinier, Y., Boukherroub, R. Inhibiting protein biofouling using graphene oxide in droplet-based microfluidic microsystems. Lab Chip. 12 (9), 1601-1604 (2012).
- Kumari, N., Garimella, S. V. Electrowetting-Induced Dewetting Transitions on Superhydrophobic Surfaces. Langmuir. 27 (17), 10342-10346 (2011).
- Freire, S. L. S., Tanner, B. Additive-Free Digital Microfluidics. Langmuir. 29 (28), 9024-9030 (2013).
- Deng, X., Mammen, L., Butt, H. -. J., Vollmer, D. Candle Soot as a Template for a Transparent Robust Superamphiphobic Coating. Science. 335, 67-70 (2011).
- Kang, K. H. How Electrostatic Fields Change Contact Angle in Electrowetting. Langmuir. 18 (26), 10318-10322 (2002).
- Abdelgawad, M., Watson, M. W. L., Young, E. W. K., Mudrik, J. M., Ungrin, M. D., Wheeler, A. R. Soft lithography: masters on demand. Lab Chip. 8 (8), 1379-1385 (2008).
- Barbulovic-Nad, I., Yang, H., Park, P. S., Wheeler, A. R. Digital microfluidics for cell-based assays. Lab Chip. 8 (4), 519-526 (2008).
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics. 77 (1), 71-94 (1974).

