Semi-High Throughput Screening for Potential Drought-tolerance in Lettuce (Lactuca sativa) Germplasm Collections
Summary
This protocol was developed to screen a large germplasm collection of the leafy vegetable lettuce (Lactuca sativa L.) for drought-tolerance, in order to identify a small candidate pool of lettuce for use in physiological, molecular, and genetic studies to identify underlying drought-tolerance traits along with breeding programs.
Abstract
This protocol describes a method by which a large collection of the leafy green vegetable lettuce (Lactuca sativa L.) germplasm was screened for likely drought-tolerance traits. Fresh water availability for agricultural use is a growing concern across the United States as well as many regions of the world. Short-term drought events along with regulatory intervention in the regulation of water availability coupled with the looming threat of long-term climate shifts that may lead to reduced precipitation in many important agricultural regions has increased the need to hasten the development of crops adapted for improved water use efficiency in order to maintain or expand production in the coming years. This protocol is not meant as a step-by-step guide to identifying at either the physiological or molecular level drought-tolerance traits in lettuce, but rather is a method developed and refined through the screening of thousands of different lettuce varieties. The nature of this screen is based in part on the streamlined measurements focusing on only three water-stress indicators: leaf relative water content, wilt, and differential plant growth following drought-stress. The purpose of rapidly screening a large germplasm collection is to narrow the candidate pool to a point in which more intensive physiological, molecular, and genetic methods can be applied to identify specific drought-tolerant traits in either the lab or field. Candidates can also be directly incorporated into breeding programs as a source of drought-tolerance traits.
Introduction
Water availability for irrigation has been a concern across much of the United States and globally for decades, but research into the response to drought-stress, along with other abiotic stresses, has lagged behind work in the areas of disease and insect resistance, at the industrial, academic, and governmental levels largely due to a lack of funding. Water availability for agriculture has historically been only an afterthought at the level of policy makers. Recently, due to several severe droughts in important agricultural production regions in both the United States and Australia1,2 fresh water availability has been thrust into the spotlight at both the national and international levels leading to many more resources being directed towards research into developing drought-tolerant cultivars of the major grain crops. While this shifting focus toward the development of drought-tolerant major crops is beneficial, like many areas of plant research specialty crops have largely been left behind.
A severe drought is currently limiting vegetable production in California, the largest production region for Lettuce (Lactuca sativa L.) in the United States3. These short-term weather patterns coupled with legislative and judicial action along with long-term climate change4,5 have combined to reduce water available for agriculture in many of the most productive regions of California. Lettuce production in California represents a 1.5 billion dollar industry accounting for nearly 80% of lettuce production in the United States3. Leafy vegetables have high leaf water content and lettuce, in particular, has a shallow root system6,7 which leaves the crop vulnerable to water-stress. In lettuce, as in all crops the development of drought-tolerant varieties will become increasingly important as fresh water supplies for irrigation become more constrained8.
This protocol lays out a method by which an initial screen was performed on a large collection of lettuce germplasm in order to identify a pool of potentially drought-tolerant candidate lines for use in physiological, molecular, and genetic studies to identify specific drought-tolerance traits. These candidates can also be used directly as sources of drought-tolerance in breeding programs to improve water use efficiency in commercial cultivars. This protocol was developed specifically to address the challenges that arise during the course of any screen of an extensive germplasm collection, especially issues of space availability and labor. Also, the protocol as presented was developed for use in lettuce, but has been successfully adapted for use in the screening of a germplasm collection of spinach (Spinacia oleracea) for potential drought-tolerance and could be modified simply to screen any leafy vegetable crop.
An important consideration before initiating a drought-tolerance screen is to understand what this method is and is not. This protocol is meant to represent a rapid method by which a large germplasm pool can be quickly and efficiently narrowed to a manageable number of candidate germplasm for use in more focused and thorough studies to identify individual tolerance traits. This protocol subjects the plants to a rapidly induced severe water-stress in contrast to a more natural slowly-induced moderate continuous drought-stress that would be observed under field conditions. The type of response induced within the plant during these two types of water-stress events (rapid dehydration versus natural drought) is not identical9 which could lead to the exclusion of some materials from future trials. These two stress events are not without overlap though10 and this protocol should serve as an effective way to identify potentially drought-tolerant germplasm contained within a large collection. This protocol alone should not be considered sufficient to identify with a high degree of certainty germplasm that contains durable drought-tolerance under field water-stress conditions, but it represents a significant step forward in the rapid screening of leafy green vegetables for potential drought-tolerance traits.
Protocol
1. Planting
- Fill plug trays (128 cell; 28 x 54 cm with cells 3 cm square and 5 cm deep) with plug soil mix. To aid in uniform filling of cells compress soil in each tray using an empty plug tray.
- Plant lettuce seed ¼ inch in depth 2-3 seeds per cell. Plant all experimental lines in replicated trays to provide both a drought stressed and control trays for the drought trial.
- Place plug trays into a base tray without holes.
- Water trays and cover with inverted tray or plastic dome. Germination normally occurs in 48-72 hr. After germination remove the cover and move trays into greenhouse.
NOTE: This protocol could also be performed in growth chambers.
2. Growth and Plant Care
- Water trays as needed from 1-4 weeks after germination, normally this is 2-3 times weekly, by filling lower tray and allowing soil to become saturated (~1 hr) rays to sit for extended periods in water as this may negatively impact plant health and root development and may affect the drought screen.
- Fertilize trays once weekly with a commercial soluble 20-20-20 fertilizer mixed at a rate of 1.5 tsp/gal.
- Thin trays to one plant per cell 1-2 weeks post germination.
3. Initiating Drought Stress
- When the plants are four weeks old separate trays into 2 groups. Subject one group to severe water stress. Use the other trays as well watered controls. Water the control group as in step 2.1 throughout the experiment. At the initiation of the drought stress suspend fertilizing all trays for the remainder of the experiment.
- The experimental drought period occurs over one week. On the initial day of the drought stress trial (Day 0) fill all lower trays with water and allow the soil in the plug trays to become fully saturated then drain water from trays. Saturating the soil immediately before the initiation of the stress period will help to minimize any variation in soil moisture between individual cells in the plug trays.
4. Conducting the Basic Drought Stress Screen with Parameters to be Measured
NOTE: The following steps outline how to collect the three physiological measurements that will be recorded over the course of the drought screen while Table 1 provides a schedule of the measurements taken on each day of the drought-stress trial period.
- Measure the Leaf Relative Water Content (RWC) on Days 0, 2, 4 of the trial.
- Clip a lettuce plant from the tray and remove 2 leaves.
- Punch 3 discs from each leaf using a #9 cork borer (~ 1.6 cm diameter). Combine leaf punches as one sample.
- Weigh the leaf discs, this is the fresh weight (FW) value used for the RWC calculation, then place the discs in a petri plate.
- Add just enough Distilled water to the plate to allow all leaf discs to float. This is to fully hydrate the leaf discs to gather turgid weights for RWC analysis. Allow the leaf discs to float for 24 hr at RT.
- Remove the now fully hydrated leaf discs from the plates and gently dry the exterior of the leaf discs with paper towels before weighing the discs. Record these weights as turgid weight (TW) for the RWC calculation.
- Place leaf discs on a paper lab wipe or filter paper disc in an open petri dish and dry in incubator at 55 °C for 24 hr.
NOTE: The paper prevents the leaf discs from adhering to the petri dish during drying. - Weigh dry leaf discs and record as dry weights (DW) for RWC calculation.
- Calculate RWC using Weatherley’s formula as described by Smart and Bingham11,12.
RWC=(FW-DW/TW-DW)*100
FW=fresh weight, TW=turgid weight, DW=dry weight - Repeat step 4.1 for each germplasm and treatment in the trial.
- Record the plant wilt once daily beginning on day 1 of the drought stress trial and ending when 100% of plants are wilted, normally day 4 or 5.
- Recovery phase and growth differential
- On day 6, conclude the drought-stress period and the stressed plants enter the recovery phase of the trial. Fill the lower trays with water and allow the drought-stressed trays to soak for 24 hr before resuming the standard watering schedule, step 2.1, as the control trays.
- Allow all plants to recover for 10 days.
- At 10 days post drought-stress harvest the entire above ground portion of each plant and record the fresh weight.
Representative Results
When performing a large screen in order to segregate a population by the desired experimental trait, in the case of this protocol drought-stress response, the data generated will correspondingly be varied from very stress susceptible to likely drought-tolerant and all points in between. Figure 1 contains graphs representing the type of results that can be expected from this protocol. Representative cultivars of three different lettuce types (romaine (cos), crisphead, and butterhead) are included in the data shown. While data representing only three lettuce types are shown here this protocol was developed during the screening of thousands of lettuce and spinach germplasm across most types. In addition to the three types of lettuce previously mentioned this protocol was successfully utilized to screen red and green leaf and stem type lettuce along with germplasm from the closely related Lactuca serriola species as well as the unrelated leafy green vegetable spinach. The authors used data generated using this protocol to narrow a lettuce collection of over 4,000 to a candidate pool of 200 lettuce varieties for field trials.
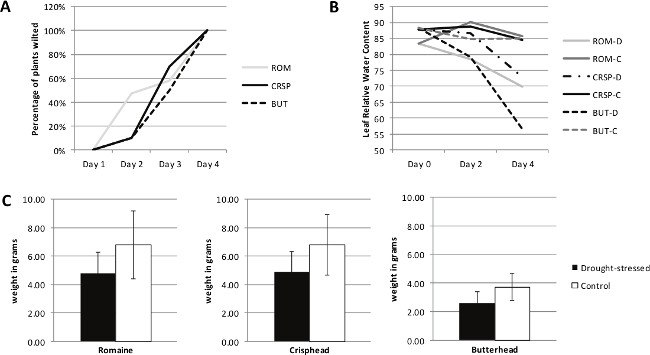
Figure 1. Representative Results Data shown is representative of the three physiological measurements included in this protocol for three lettuce types, including romaine (cos, ROM), crisphead (CRSP), and butterhead (BUT). (A) The percentage of plants that are wilted on each day of the drought-stress period of the trial, starting on day 1, is recorded until all plants reach 100% wilted. (B) Leaf relative water content is measured on days 0, 2, and 4. –D: drought-stress, -C: control. (C) Weight of plants after drought-stress period and 10 day recovery showing growth differential due to drought-stress. Error bars represent standard deviation. Please click here to view a larger version of this figure.
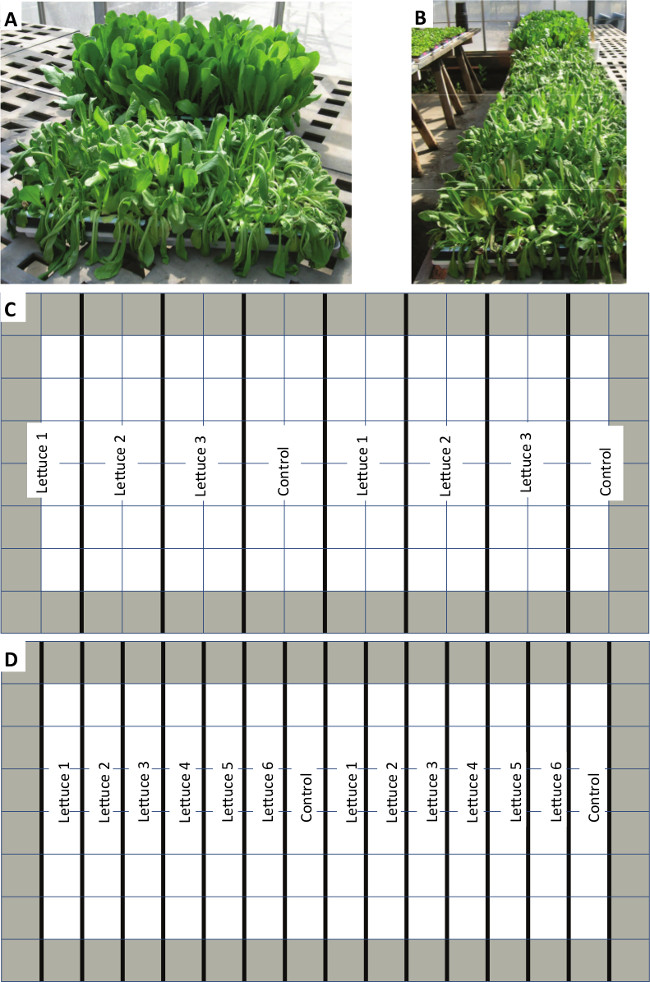
Figure 2. Representative trays and example planting diagrams (A and B) Example trays show the drought-stress response on day 3 of a trial using this protocol as compared to the control trays. (C and D) These tray diagrams represent the 128 plug trays used showing two planting methods utilized in this protocol. The outer edges of the trays (gray shaded cells) should be planted, but not utilized for experimental samples, due to the propensity of the edge cells to dry more rapidly than interior cells.
| Day | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| RWC-FW | X | X | X | ||||
| Wilt | X | X | X | X | (X) | (X) | |
| Withhold water | X | ||||||
| Resume watering | X |
Table 1. Timetable of drought-stress period. Water is withheld from the experimental trays beginning on day 0 of the trial and watering resumes on day 6. Fresh samples for leaf relative water content measurements are taken on days 0, 2, 4, and the percentage of plants wilted are recorded daily beginning on day 1 and continuing until 100% of plants are wilted, normally day 4, but may extend to day 5 or even 6.
Discussion
Considerations of sample number for screen.
The number of samples needed should be based on the desired use of the data from this screen. If publication quality results are desired it is recommended to harvest 3 individual plants (3 biological replicates) from each line and perform a minimum of 2 experimental replicates to give adequate points for quality statistical analysis. If the desired outcome is simply to quickly narrow a large pool of candidate germplasm in order to perform more stringent or complex water-stress experiments, less samples and or replicates may be necessary. The number of samples required must be decided at the time of planting.
Planting layout and considerations of soil drying in trays.
One very important consideration is that the soil in cells on the edge of the plug tray will dry more rapidly than interior cells and care should be taken to avoid bias in results by only harvesting plants from the tray interior (Figure 2). Only harvesting plants from the interior cells will likely influence the planting layout of the tray. During the screen in which this protocol was developed between 3 and 6 unique germplasm was planted per plug tray along with a control lettuce cultivar. One of the challenges of screening for drought tolerance in a specialty crop is often there exists no know stress tolerant or susceptible germplasm to use as positive or negative controls. When no positive or negative control is available as in the development of this protocol the use of a standard germplasm throughout all trials allows for a basic level of internal control for variability of conditions throughout each screen. The number of different germplasm that can be planted will directly contribute to the speed at which a germplasm collection can be screened, but is limited by the number of plants needed for each measurement taken during the course of the trial. By limiting the number of unique germplasm in each tray to between 3 and 6 all lettuce can be planted in two locations within each tray assuring that no lettuce is planted in only exterior cells. Another consideration is that variability in growth rate and plant size amongst germplasm may lead to reduced rates of soil drying in cells containing small varieties. This could potentially bias results in the direction of small varieties being over represented among potential drought-tolerant germplasm pulled from this screen, but this did not seem to be the case based on the observed results. Furthermore, the use of stringent secondary methodologies to study the candidate germplasm from this protocol should either confirm or refute these results.
The Importance of consistency on the observation of wilt.
The monitoring of plants for wilt should be performed at the same time daily to avoid small fluctuations in the appearance of plant stress, observed as wilt, likely caused by temperature and humidity fluctuations present in a greenhouse as well as circadian regulation of stomata. Also, be sure to be consistent when scoring plants as wilted or not. Establishing a set of guidelines in scoring wilt is especially important if multiple individuals will be performing the screen to assure consistent results. The threshold used for this protocol is all leaves of the plant must be wilted to score the plant as being wilted.
Selection of parameters measured.
The parameters measured in this protocol were chosen based on their usefulness in identifying water stress along with the adaptability of the measurements to a semi-high throughput system utilizing minimal available labor. Many other measurements can be very helpful in identifying drought-stress (e.g., photosynthetic activity, root growth, stomatal conductance…), and based on the nature of the screen that will be performed (i.e., number of germplasm used, number of replicates, rate of throughput desired…) other parameters can easily be incorporated into this screen.
Comments on the use of protocol results.
The USDA collections contains over 4,000 individual lettuce germplasm which is a number that makes field trials incorporating all germplasm impractical. This protocol was developed with the lone purpose of allowing a very small number of researchers (1-2 individuals) to screen the USDA collections for potential drought tolerance. Through the use of these methods the USDA collection was narrowed to 200 varieties which were then used in field trials that can more closely replicate drought-stress conditions. This protocol was also used to narrow a population of over 400 spinach germplasm to 40 for use in field trials. This protocol alone should not be considered sufficient to identify with a high degree of certainty germplasm that contains durable drought-tolerance under field water-stress conditions, but can simply serve as a tool to rapidly screen for potential stress tolerance under water deficit conditions in a rapid and efficient screen utilizing large numbers of germplasm.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank California Department of Food and Agriculture for funding of the project which led to the development of this protocol. CDFA SCB11019.
Materials
| Name of Material/ equipment | Manufacturer | Catalog number | Comments |
| plug tray 128 | T.O. Plastics Hummert International |
11-8595-1 | Any brand plug tray will work, but use the same style of trays for all trials. |
| lower tray (Display tray) | T.O. Plastics Hummert International |
11-3305-1 | |
| plug/planting mix (Sunshine Mix #5) | Sunshine Hummert International |
10-0467-1 | A different mix may need to be substituted if adapting this protocol to a different crop. Sunshine mix #4 was used in spinach trials. |
| fertilizer (20-20-20) | Jack's: Professional water-soluble fertilizer Hummert International |
07-5915-1 | Any fertilizer can be used, adjust type as needed for adapting this protocol to specific crop needs. |
References
- Dijk, A. I. J. M., et al. The Millennium Drought in southeast Australia (2001–2009): Natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resour. Res. 49, (2013).
- Aghakouchak, A., Feldman, D., Stewardson, M. J., Saphores, J., Grant, S., Sanders, B. Australia’s Drought: Lessons for California. Science. 343, 1430 (2014).
- Tolomeo, V. California Agricultural Statistics 2012 Crop Year. USDA National Agricultural Statistics Service Pacific Regional Office-California. , (2013).
- Cavagnaro, T., et al. Climate Change: Challenges and Solutions for California Agricultural Landscapes. White paper CEC-500-2005-189-SF. California Climate Change Center. , (2005).
- Lobell, D. B., Gourdji, S. M. The Influence of Climate Change on Global Crop Productivity. Plant Physiol. 160, 1686-1697 (2012).
- Jackson, L. E., Stivers, L. J. Root distribution of lettuce under commercial production: implications for crop uptake of nitrogen. Biological Agriculture and Horticulture. 9, 273-293 (1993).
- Jackson, L. E. Root architecture in cultivated and wild lettuce (Lactuca spp). Plant. Cell and Environment. 18, 885-897 (1995).
- Malcom, S., Marshall, E., Aillery, M., Heisey, P., Livingston, M., Day-Rubenstein, K. Agricultural Adaptation to a Changing Climate: Economic and Environmental Implications Vary by U.S Region. USDA Economic Research Service. Economic Research Report Number. 136, (2012).
- Chaves, M. M., Maroco, J. P., Pereira, J. S. Understanding plant responses to drought- from genes to the whole plant. Funct. Plant Biol. 30, 239-264 (2003).
- Ingram, J., Bartels, D. The Molecular Basis of Dehydration Tolerance in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 377-403 (1996).
- Weatherley, P. E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phlytol. 49, 81-97 (1950).
- Smart, R. E., Bingham, G. E. Rapid Estimates of Relative Water Content. Plant Physiol. 53, 258-260 (1974).

