Neural Activity Propagation in an Unfolded Hippocampal Preparation with a Penetrating Micro-electrode Array
Summary
We have developed an in vitro unfolded hippocampus which preserves CA1-CA3 array of neurons. Combined with the penetrating micro-electrode array, neural activity can be monitored in both the longitudinal and transverse orientations. This method provides advantages over hippocampal slice preparations as the propagation in the entire hippocampus can be recorded simultaneously.
Abstract
This protocol describes a method for preparing a new in vitro flat hippocampus preparation combined with a micro-machined array to map neural activity in the hippocampus. The transverse hippocampal slice preparation is the most common tissue preparation to study hippocampus electrophysiology. A longitudinal hippocampal slice was also developed in order to investigate longitudinal connections in the hippocampus. The intact mouse hippocampus can also be maintained in vitro because its thickness allows adequate oxygen diffusion. However, these three preparations do not provide direct access to neural propagation since some of the tissue is either missing or folded. The unfolded intact hippocampus provides both transverse and longitudinal connections in a flat configuration for direct access to the tissue to analyze the full extent of signal propagation in the hippocampus in vitro. In order to effectively monitor the neural activity from the cell layer, a custom made penetrating micro-electrode array (PMEA) was fabricated and applied to the unfolded hippocampus. The PMEA with 64 electrodes of 200 µm in height could record neural activity deep inside the mouse hippocampus. The unique combination of an unfolded hippocampal preparation and the PMEA provides a new in-vitro tool to study the speed and direction of propagation of neural activity in the two-dimensional CA1-CA3 regions of the hippocampus with a high signal to noise ratio.
Introduction
Understanding the neural conduction or propagation of neural signals is crucial for determination of the mechanism of neural communication in both the normal function and pathological conditions in the brain 1-3. The hippocampus is one of the most extensively studied structures in the brain since it plays fundamental role in several brain functions such as memory, and spatial tracking and is involved in several pathological changes that dramatically impact behavior as well 1,6 . Although, the hippocampus exhibits a complex organization, the different elements of its structure can be readily identified and accessed in the slice preparation4-6. In the transverse direction of the hippocampus, neural activity is known to propagate through the tri-synaptic pathway that comprise the Dentate Gyrus (DG), CA3, CA1 andsubiculum 4,5. It is believed that synaptic transmission and axonal conduction play a major role for communication in this transverse circuit 4,6. However, propagation of neural signal takes place in both transverse and longitudinal directions 4,6. This implies that the hippocampus cannot be fully investigated by using slice preparations which limit the observation to a particular direction of propagation 4. The longitudinal slice was developed to investigate the axonal pathways along the longitudinal axis 5. Researchers have observed behavior-specific gamma and theta oscillations predominantly along the transverse and longitudinal axes respectively 6. These behaviors have been studied separately, yet simultaneous access to both directions is crucial to understand these behaviors. Even with the development of the intact hippocampus preparation, it is difficult to monitor the propagation throughout the entire tissue due to the folded-structure of the hippocampus 4. The unfolded hippocampus provides access to the packed neurons in a form of a flat two-dimensional cell layer 7,8.
By unfolding the dentate gyrus (DG) (Figure 1), the hippocampus adopts a flattened shape with a rectangular configuration in which both transverse and longitudinal connections remain intact with the pyramidal cell layer arranged in a two-dimensional sheet containing both CA3 and CA1, leaving a flat piece of neural tissue that can be used to investigate neural propagation (Figure 2) 8. Neural activity can then be monitored with individual glass pipettes, microelectrode arrays, stimulating electrodes, as well as voltage sensitive dyes (VSD) 3,7,8. In addition, genetically encoded voltage indicator from transgenic mice can be used to track the propagation pattern 9.
The flat configuration of the unfolded hippocampal network is well suited for optical method recording but also for a microelectrode array. Most of the commercially available arrays are fabricated with flat or low profile electrodes and can record neural activity in both tissue slices and cultured neurons 10-12. However, the signal-to-noise ratio (SNR) decreases when the signals are obtained from an intact tissue since the soma of the neurons are located deeper into the tissue. Microelectrode electrode arrays with high aspect ratios are required to improve the SNR.
To this effect, a penetrating microelectrode array (PMEA) has been developed in our laboratory, and provides the ability to directly probe into the tissue by inserting 64 spikes with a diameter of 20 µm and height of 200 µm into the unfolded hippocampus 7,13. This microelectrode array has higher SNR compared to the voltage sensitive dye imaging and the SNR remains stable during an experiment 7,13. The combination of the unfolded hippocampal preparation and the PMEA provides a new way to investigate the neural propagation over a two-dimensional plane. Experiments using this technique have already yielded significant results about the mechanisms of neural signal propagation in the hippocampus whereby neural activity can propagate independently of synaptic or electric synapses 7.
Protocol
NOTE: Animal experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee at the university. CD1 mice of either sex at the age of P10 to P20 are used in this study.
1. Solutions for Surgery and Experimental Recording
- Prepare normal artificial cerebrospinal fluid (aCSF) buffer containing (mM): NaCl 124, KCl 3.75, KH2PO4 1.25, MgSO4 2, NaHCO3 26, Dextrose 10, and CaCl2 2. Use this normal aCSF for tissue recovery after dissection, as well as for washing system at the beginning of the experiment.
- Prepare sucrose aCSF that is used during the hippocampus dissection and contains (mM): Sucrose 220, KCl 2.95, NaH2PO4 1.3, MgSO4 2, NaHCO3 26, Dextrose 10, and CaCl2 2. In order to induce epileptiform activity in the intact hippocampal tissue preparation, add 4-Aminopyridine (4-AP) to normal aCSF at the concentration of 100 µM.
2. Surgical Procedure for the Intact Hippocampus Preparation
- Drop isoflurane (1 ml) into the chamber at the bottom of a desiccator glass jar (No.1 in Specific Materials and Equipment) and use a regular paper towel to cover the surface of the bottom stage so the animal does not get in contact with the liquid. Then, place the CD1 mouse into the jar and close the lid. Keep the animal in the closed jar for about 1 min to 2 min. When breath frequency is about one per second, remove the mouse from the jar.
- Place the mouse on the surgery stage and decapitate with a suitable scissors. Immediately after decapitation, place the head into the ice-cold (3-4 °C), oxygenated sucrose aCSF for about 30 sec.
- Use fine scissors (No.5 in Specific Materials and Equipment) to remove the skin on top of the skull and to cut the skull along the middle line of the head as well as at two ends near the temporal lobe. Use forceps (No. 6 in Specific Materials and Equipment) to peel the cut skull towards each side of the head in order to expose the brain.
- Insert a micro lab spatula (No. 8 in Specific Materials and Equipment) into the gap between the parietal lobes of the brain and skull to carefully peel the brain from the bottom of the skull and then drop it onto a prepared ice-cold (3-4 °C) surgical stage covered with wet filter paper (No.2 in Specific Materials and Equipment). Remove the cerebellum with an ice-chilled blade and separate the two hemispheres by cutting the midline of the brain. Then, place the two separated hemispheres into a beaker filled with the ice-cold sucrose aCSF bubbled with 95% O2/ 5% CO2.
- Take one half of the hemisphere and place on the ice-cold filter paper stage. Place regular paper towels or filter paper around the brain to suck the extra solution. Use two fire-polished glass pipette tools (No. 10 in Materials and Equipment) to separate the cortex from the rest of the central part of the brain.
- After the hippocampus is exposed from inside of the cortex and put on the ice-cold stage, place two or three drops of ice-cold sucrose aCSF on the tissue and then remove extra solution around the hippocampus. Cut the connections with the cortex at two ends of the hippocampus. Then, dissect the hippocampus out of the brain with the fire polished glass tools and remove the remaining part of the brain.
- Separate the whole hippocampus with its alveus side facing up and hippocampal sulcus facing down. Quickly drop two or three drops of ice-cold sucrose aCSF on the tissue again and remove the extra solution around the tissue using a piece of paper towel. Use a fire polished glass tool to turn over the whole hippocampus to expose the sulcus (Figure 1B, C).
- Under a normal optical microscope, insert a custom made glass needle (No. 11 in Specific Materials and Equipment) into one end of the sulcus and cut the fiber connections, from dentate gyrus (DG) to subiculum or the CA1 field, along the direction of sulcus (Figure 1C). Apply an ice-cold blade to trim the septal and temporal ends of the hippocampus if necessary. Insert a custom made metal wire loop (No. 12 in Specific Materials and Equipment) into the cut sulcus and pull over the DG away from the tissue while holding the subiculum/CA1 end of the hippocampus by a fire polished glass tool (Figure 1D).
- Following the unfolding procedure above, add another two or three drops of the ice-cold sucrose aCSF onto the tissue and remove the extra solution around the tissue. Then, trim the unfolded hippocampus with the ice-cold blade at the edges (Figure 1E) and place the preparation by a spatula into the recovering chamber filled with normal aCSF and bubbled with 95% O2/ 5% CO2 at RT (about 25 °C). Leave the unfolded hippocampus tissue to recover about 1 hr before placing it into the recording chamber.
- Take the other brain hemisphere and use it to go through steps from 2.5 to 2.9. Usually, take about 1 to 2 min to finish the whole procedure to unfold a single hippocampus and drop ice-cold sucrose aCSF continuously to keep the tissue oxygenated and hydrated.
3. Experimental System Setup
- Glue a custom fabricated PMEA on a pin grid array (PGA) package and use micro wire bonding to connect each pad from a single microelectrode to the pad of a pin on the package (Figure 3B). Then, insert the package into the socket on a custom-made circuit board 13.
- Individually connect each microelectrode to its filters with band pass cut-off frequency from 1 Hz to 4 KHz and amplifiers with gain of 100 on the custom made circuit board 13. Digitize the analog output from the circuit board by an A/D converting system, and acquire and store the data on a computer.
- Glue a custom-made plastic recording chamber around the array with inlet and outlet tubing for the solution flow (Figure 4A). Use at least two bottles to keep different solutions in the system, and join and control the output tubing of the bottles by a tri-valve. Connect the output of the tri-valve to a tubing system with an IV drip chamber which is guiding the solution into the recording chamber.
- Between the tri-valve and the inlet of the recording chamber, add an electrical heater to heat the solution to a controlled temperature (35 °C) before the solution is guided to the recording chamber. Attach the outlet of the recording chamber to a vacuum tube to collect the solution into a vacuum tube connected flask. Do not recycle any solution in any experiment in this study.
4. Placing the Unfolded Hippocampus onto the PMEA to Record the Neural Activity
- To prepare the experimental setup, fill one bottle with normal aCSF and another bottle with 4-AP aCSF. In both bottles, bubble 95% O2/ 5% CO2 from the very beginning of each experiment. Use a tri-valve connector to control which solution will be selected during an experiment. Connect a vacuum tube at the outlet of the chamber to pump the solution into a dust container. Heat the pipeline before delivering it into the recording chamber and keep the solution at a controlled temperature level (35 °C).
- When the inlet and outlet of recording chamber are closed, use a custom made glass pipette dropper to transfer and place the unfolded hippocampus into the recording chamber. Under the microscope, position the unfolded hippocampus using a regular small paint brush while the tissue is floating in the solution. Place the unfolded hippocampus with its alveus side facing down, CA3 area pointing away, and CA1 field pointing towards the researcher.
- Carefully suck away the solution in the chamber using a vacuum pipette from the edge of the recording chamber to lower the solution level until the chamber is dried and the tissue is lowered onto the array. Then, carefully place a custom made tissue anchor (Figure 4) (No. 14 in Specific Materials and Equipment) on top of the tissue to hold the unfolded hippocampus onto the array. Put a few drops of solution into the recording chamber to refill it, and gradually open the inlet and outlet to adjust flow rate to about two drops per second in the IV drip chamber.
- Incubate the tissue in the recording chamber with normal aCSF for about 1 min to recover, then switch the solution supply to 4-AP dissolved aCSF and adjust the flow rate properly. Incubate the tissue in 4-AP dissolved aCSF for about 5 to 10 min and then the researcher could start the software to record the signal when spontaneous activity appears.
5. Removing the Tissue from the PMEA After an Experiment
- Control the tissue anchor by a micromanipulator and gradually lift the tissue from the recording chamber. Shut down both inlet and outlet to stop the flow in the recording chamber. The recording chamber should be full with solution or add a few drops of solution to fill the chamber if the recording chamber is not full.
- Use a small paint brush to lift each corner of the tissue. If the tissue is not floating in the solution, then employ the vacuum tube to dry the chamber carefully with the tissue still sitting on the array. Then carefully open the inlet to gradually refill the chamber and shut down the inlet to stop the flow when the recording chamber is full. Apply the small paint brush to lift each corner of the tissue again.
- Repeat step 5.2 until the tissue is detached from the array and floating in the solution. If the tissue is floating in the solution, then use the vacuum tube to suck the tissue away. Open the flow in the inlet and open the vacuum in the outlet. Wash the system with distilled water and dry it out.
Representative Results
The data shown in the figures here were recorded in the unfolded hippocampus preparation with 4-AP (100 µM) aCSF added during incubation of the tissue in the recording chamber at RT (25 °C). Normally activity starts within 5 min, but in some hippocampal tissues from the older animals it may take longer. The 4-AP-induced neuronal firing observed with the PMEA is the same as previously reported 14,15. Since the electrodes have a height of 200 µm, the electrode tips are located just below the cell layer (Figure 3C) because the cell layer is usually 250 to 300 µm above the alveus of the hippocampus(Figure 2), recordings (57 out of 64 in the sample experiment) from different channels have a mostly positive deflection. However, some of these positive recordings could display small negative deflections as well (Figure 5B) if the recording electrode tips are close enough to the cell layer. If the electrode tips are located right at the level of cell layer, the recording will have very sharp negative spiking with positive shift on it or just negative spiking (Figure 5C) 16. In the data sample shown here, 5 channels out of 57 recordings have negative spiking. After acquiring the data from all the 64 channels, the method of individual normalization (Figure 6B) is applied to map the neural propagation on a 2-D plane along the time axis of the recording 7. With a combination of the PMEA and the unfolded hippocampus, neural propagation is mapped and observed to initiate mostly in one side of CA3 and move longitudinally in a diagonal wave front, crossing the entire area of the hippocampus (Figure 6).
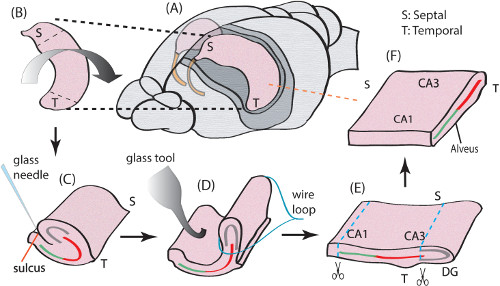
Figure 1. Surgical procedure for an unfolded hippocampus. (A) One of the two hippocampi is dissected from temporal lobe of a mouse brain. (B) Septal and temporal terminations along the longitudinal axis. (C) The hippocampus is flipped over with a fire polished glass pipette to expose the sulcus. Both ends of the hippocampus are trimmed and a glass needle is used to cut the connections between DG and CA1 or subiculum along the longitudinal axis. (D) The hippocampus is unfolded by a custom-made metal wire loop. (E) Unfolded hippocampus with subiculum and DG trimmed. (F) The final tissue preparation of an unfolded hippocampus. This position shows the orientation of the hippocampus when it is placed on the array in an experiment. For additional details about the unfolded hippocampus anatomy please refer to experimental methods in previously published manuscripts 7. Please click here to view a larger version of this figure.
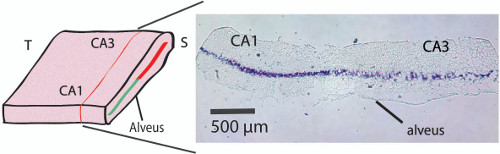
Figure 2. Histology cross-section of the unfolded hippocampus. Crystal-violet stain shows the position of the CA1-CA3 pyramidal cell layer within the unfolded hippocampus a level of 250 to 300 µm above the alveus thereby locating the microelectrodes right below the cell layer (refer to Figure 3C). To obtain the sections stained with the crystal violet, the tissue was post-fixed after the hippocampus was unfolded. The unfolded hippocampus was placed in PFA 4% O/N. Then, the tissue was transferred and kept in sucrose solution (30%) for 48 hr, and followed by snap-freezing in a biker containing isopentane (2-Methylbutane) to cool down to -35 °C in dry ice. Frozen section were then cut in a cryostat with 20 µm thick sections in the transverse plane (as shown in the Figure 2) to reveal the location of the pyramidal cell layer. Please click here to view a larger version of this figure.
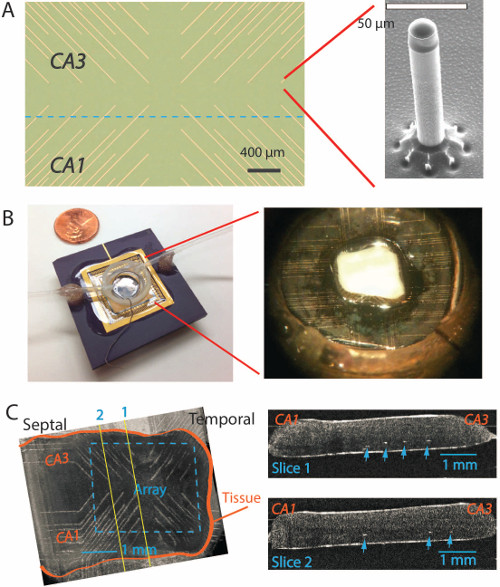
Figure 3. Unfolded hippocampus on the PMEA. (A) Top view of a new PMEA with the microelectrodes located at the end of each gold metal trace. The insert on the right side shows a single microelectrode under electron microscopy with a height of 200 µm and a diameter of 20 µm 7,13. (B) The microelectrode array is glued on a PGA package with a plastic recording chamber around the microelectrodes on a glass substrate. The insert on the right shows an unfolded hippocampus placed on the microelectrode array in the middle. (C) On the left, Optical Coherence Tomography(OCT) imaging shows the unfolded tissue positioned on top of the array in a different experiment. On the right, two longitudinal slice images obtained from the OCT imaging on the left shows the microelectrode tips (white dots pointed by the arrows) reach into the area right below the cell body layer. Please click here to view a larger version of this figure.
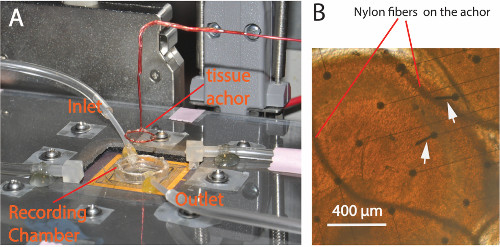
Figure 4. Experimental setup. (A) A plastic cover lid with screws is placed over the circuits to protect it against possible water damage. The recording chamber has both inlet and outlet tubes to carry the solution flow. A custom made tissue anchor glued with a Nylon fiber mesh is used to secure the tissue during the experiments. (B) Picture taken from the bottom of the glass substrate of the PMEA showing the tissue anchor holding a sample slice during an experiment. The curved wires are the Nylon fibers from the mesh pressing on top of the tissue. The round dots are the bases of microelectrodes. Arrows point to electrodes that were damaged after several experiments. Please click here to view a larger version of this figure.
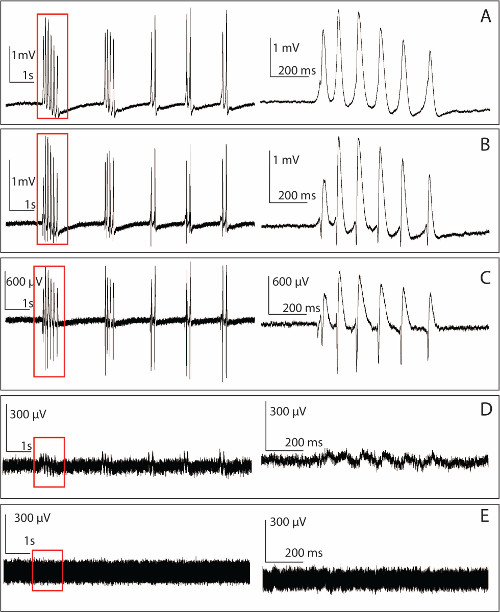
Figure 5. Raw data recorded by the PMEA from an unfolded hippocampus. Left panel: 4-AP-induced epileptiform activity recorded from a single microelectrode. Right panel: Zoomed-in version of the signal marked in the time window on the left. (A) 10 sec section of an example of the spontaneous activity induced by 100 µM 4-AP aCSF obtained for one of the microelectrodes located in the basal dendritic region based on the polarity of the signals with a SNR of 34.9 dB. (B) Raw data from another microelectrode located closer to the somata with a SNR of 27.2 dB. (C) Example of recording obtained from an electrode positioned within the somatic layer. In this example, the SNR is 18.53 dB. (D) Recording obtained from a bent electrode still conducting but not penetrating into the tissue. The bent electrode has a significant lower SNR compared to those intact electrodes (1.5 dB in this example). (E) Baseline noise recorded from a microelectrode. The baseline usually has a peak to peak value from 150 to 200 µV and the impedance of a single electrode is around 1 to 2 MΩ. Please click here to view a larger version of this figure.
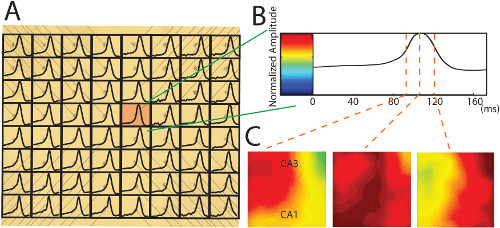
Figure 6. Conversion of neural recording data into the 2-D propagation maps. (A) A 170 msec time window truncates a single neural spiking in each channel plotted on the photo of the PMEA. The raw data is filtered by a low pass filter at 100 HZ. The signals from the broken electrodes are interpolated with the recordings around it. In this example, all the spikes are positive. (B) A single neural spike from the red pixel in (A) is normalized to the color bar with a custom-developed method for individual normalization (Please refer to the previous publication for more details about the normalization 7). (C) The color maps created by the individual normalization show that propagation moves across the entire area of the unfolded hippocampus at different time points. Please click here to view a larger version of this figure.
Discussion
The development of the unfolded hippocampus preparation, where the longitudinal and transverse axes of the hippocampus are preserved in combination with a penetrating microelectrode array, provides a powerful tool to investigate the anatomy connections or neural propagation in the hippocampus 7. This unfolding procedure is also applicable for studying hippocampus in adult mice. Recent studies with this preparation showed that the 4-AP-induced epileptiform activity could propagate with a diagonal wave front across the entire area of the unfolded hippocampus (Figure 6) 7,8. These studies showed that the intact unfolded hippocampus provides significant advantages over either transverse or longitudinal slices when the neural propagation in a flat hippocampal neural network can be investigated (Figure 6).
The penetrating microelectrode provides a significant advantage over existing microelectrode arrays (MEA) which consist of multiple contacts positioned over a flat surface 11,12,17. MEA with flat surface electrodes are difficult to use with the unfolded hippocampus since the cell layer is located about 250 to 300 µm away from the surface (Figure 2). The PMEA described here was designed to solve this problem with 64-penetrating electrodes suitable for studying the neural propagation with a height of 200 µm to reach deep inside the tissue 7,13. In addition, the PMEA also applicable in any flat tissue preparation, such as cortical slices, hippocampal transverse slices etc.
Another advantage of using this PMEA is the improved SNR. A prototype study of this PMEA shows the SNR ratio of neural signal recorded by this PMEA has an average value of 19.4 ± 3 dB, which is significantly higher and more stable compared to that of VSD recording since previous studies indicated that the toxicity and photo bleaching of VSD clearly impaired the ability to record data 8. With an improved experimental protocol by using the tissue anchor in this study, the SNR of the recording from the PMEA increases to a range from about 20 to 30 dB (Figure 5), which has a higher SNR compared to those flatten-electrode arrays with a value from 10 to15 dB 11,18. The ability to unfold the hippocampus is essential in order obtain a layer of neurons (CA1-CA3) that can be interrogated by a flat recording array.
Furthermore, since the silicon array is built on a transparent substrate, voltage sensitive dye imaging technique can be integrated in the PMEA system to study the propagation of neural activity in the hippocampus 8. Transgenic mice with fluorescent voltage sensitive indicators can also be used to investigate the origin and propagation of the signal under physiological conditions as well as changes induced by pharmacological agents or genetic modified tissue from various animal models 9.
There are several critical steps in order to assure high quality recording. Firstly to ensure tissue viability, the surgical procedure must be carried out as quickly as possible. Usually, it takes about 1 to 2 min to go through all the steps from 2.1) to 2.10). Practice of the unfolding procedure on some sample animals is highly suggested before actual experimental surgery is carried out. Secondly, the flow rate needs to be kept constant to avoid any fluctuation of the level of the perfusion fluid in the recording chamber. Furthermore, the tissue should be anchored down to avoid movement of the tissue relative to the array.
Although the PMEA described here can provide useful signals in monitoring the neural propagation in the unfolded hippocampus, there are some drawbacks and limitations to this recording methodology.
Firstly, the microelectrodes can break due to the mechanical force placed on them (Figure 5D, E). During the procedure of tissue placement and removal, the accidental contact between the small paint brush and array could cause the microelectrodes to bend or break. When the tissue anchor is lowered down by the micromanipulator, the horizontal movement of the tissue along the bottom of the chamber could create a shear force that could lead to bending or breakage of the electrodes. In a future design, the microelectrodes should be reshaped with a somewhat thicker base to make them stronger. In the current version of this PMEA, the recording electrodes have a narrow base compared to its diameter 13, thereby weakening the electrodes (Figure 3A).
The cleaning procedure should also be improved to adequately remove the residual tissue and fibers that could attach to the array. After each experiment, small pieces of tissue could remain attached to the microelectrodes and this residual tissue left on the electrodes must be removed. If these residual tissues are not removed, the impedance of the electrodes and the SNR of the recordings could be affected. Flushing of the system with distilled water is suggested as indicated in part 5 of the Protocol section. Users are also advised to flush the system with some weak acidic solution which can dissolve the tissue without damaging the system
Another drawback of this protocol is that during the unfolding procedure, the perforant path in this unfolded hippocampus is cut, preventing investigation of the possible neural signals propagating from DG to the other layers of the hippocampus.
In conclusion, we have described here a novel methodology to investigate the propagation of neural activity within the hippocampus by combining a high aspect ratio microelectrode array with unfolded hippocampal tissue. The method did lead to a better understanding of how neuronal activity can propagate in both the longitudinal and transverse directions. This technique could also be applied to cortical tissue placed in a similar way on top of the array. Moreover, combining optical recording technique with this preparation is possible since the array is transparent and could also lead to some important findings about signal propagation in neural tissue.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by National Institutes of Health (National Institute of Neurological Disorders and Stroke) Grant 1R01NS060757-01 and by the E.L. Lindseth endowed chair to Dominique M. Durand. We thank Dr. Andrew M. Rollins’ laboratory for the help on the OCT imaging.
Materials
| desiccator jar | LABRECYCLERS Inc. | 5410 | Place regular paper towels at the bottome of the jar for animal anesthesia use. |
| A blade and Custome made surgical stage for unfolding hippocampus | N/A | N/A | A petri dish is place upside down (in the center) in the ice with a wet filter paper place on top of it. |
| Custom made tissue recovery chamber | N/A | N/A | Plastic tubes were glued with plastic mesh at the bottom and bubbled with 95% O2/ 5% CO2 in the aCSF. |
| Straight Operating Scissors | Fisher Scientific | S17336B Medco Instruments No.:81995 | This scissors is used to decapitate the mice. |
| Integra Miltex Goldman-Fox Scissors | Fisher Scientific | 12-460-517 MILTEX INC No.:5-SC-320 | This scissors is used to cut the skull of the mice. |
| Miltex Hysterectomy Forceps |
Claflin Medical equipment | CESS-722033-00001 | This Forceps is used to peel the cut skull to expose the brain |
| Micro Spatula | Cardinal Health | This micro spatula is used to tranfer the whole brain of a semisphere into the recorering chamber. | |
| Frey Scientific Stainless Steel Semi-Micro Spatula | Cardinal Health | this semi micro spatula is used to tranfer the unfolded hippocampus into the glucose aCSF in the recovering chamber. | |
| small paint brush | Lowe's | tem #: 105657 Model #: 90219 | The one with the smallest size in a normal paint brush package |
| Fire polished glass help tool | N/A | N/A | This tool was fire polished and made from the regular Pasteur glass pipettes. |
| Custom made glass needle | N/A | N/A | This tool was fire polished and made from the regular Pasteur glass pipettes. |
| Custom made glass tool with a metal wire loop | N/A | N/A | This tool was fire polished and made from the regular Pasteur glass pipettes with a reshaped metal wire loop. |
| Custom made glass solution dropper | N/A | N/A | This tool was made from the regular Pasteur glass pipettes with its tips cut and a rubber head attached with the cut end. |
| Custom made tissue anchor | N/A | N/A | Nylon fiber mesh was glued on a insulated copper wire ring. The tissue anchor was hold by an micromanipulator. |
| Custom fabricated microelectrode array | N/A | N/A | More detail about the array please refer to Kibler, et al, 2011. |
| Custom made filter and amplifiers circuits for the array | N/A | N/A | More detail about the array please refer to Kibler, et al, 2011. |
| Data acquisition processor 3400a | Microstar Laboratories | N/A | This is a complete data acquisition system with A/D converter. |
References
- Richardson, K. A., Schiff, S. J., Gluckman, B. J. Control of traveling waves in the Mammalian cortex. Phys Rev Lett. 94 (2), 028103-028112 (2005).
- Luhmann, H. J., Dzhala, V. I., Ben-Ari, Y. Generation and propagation of 4-AP-induced epileptiform activity in neonatal intact limbic structures in vitro. Eur J Neurosci. 12 (8), 2757-2768 (2000).
- Grinvald, A., Manker, A., Segal, M. Visualization of the spread of electrical activity in rat hippocampal slices by voltage-sensitive optical probes. J Physiol. 333, 269-291 (1982).
- Gloveli, T., et al. Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc Natl Acad Sci USA. 102 (37), 13295-13300 (2005).
- Amaral, D. G., Witter, M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 31 (3), 571-591 (1989).
- Albani, S. H., McHail, D. G., Dumas, T. C. Developmental studies of the hippocampus and hippocampal-dependent behaviors: insights from interdisciplinary studies and tips for new investigators. Neurosci Biobehav Rev. 43, 183-190 (2014).
- Zhang, M., et al. Propagation of Epileptiform Activity Can Be Independent of Synaptic Transmission, Gap Junctions, or Diffusion and Is Consistent with Electrical Field Transmission. J Neurosci. 34 (4), 1409-1419 (2014).
- Kibler, A. B., Durand, D. M. Orthogonal wave propagation of epileptiform activity in the planar mouse hippocampus in vitro. Epilepsia. 52 (9), 1590-1600 (2011).
- Wang, D., McMahon, S., Zhang, Z., Jackson, M. B. Hybrid voltage sensor imaging of electrical activity from neurons in hippocampal slices from transgenic mice. J Neurophysiol. 108 (11), 3147-3160 (2012).
- Wingenfeld, K., Wolf, O. T. Stress , memory, the hippocampus. Front Neurol Neurosci. 34, 109-121 (2014).
- Liu, J. S., et al. Spatiotemporal dynamics of high-K+-induced epileptiform discharges in hippocampal slice and the effects of valproate. Neurosci Bull. 29 (1), 28-36 (2013).
- Oka, H., Shimono, K., Ogawa, R., Sugihara, H., Taketani, M. A new planar multielectrode array for extracellular recording: application to hippocampal acute slice. J Neurosci Methods. 93, 61-68 (1999).
- Kibler, A. B., Jamieson, B. G., Durand, D. M. A high aspect ratio microelectrode array for mapping neural activity in vitro. J Neurosci Methods. 204 (2), 296-305 (2012).
- Schechter, L. E. The potassium channel blockers 4-aminopyridine and tetraethylammonium increase the spontaneous basal release of [3H]5-hydroxytryptamine in rat hippocampal slices. J Pharmacol Exp Ther. 282 (1), 262-270 (1997).
- Perreault, P., Avoli, M. 4-aminopyridine-induced epileptiform activity and a GABA-mediated long-lasting depolarization in the rat hippocampus. J Neurosci. 12 (1), 104-115 (1992).
- Chesnut, T. J., Swann, J. W. Epileptiform activity induced by 4-aminopyridine in immature hippocampus. Epilepsy Res. 2 (3), 187-195 (1988).
- Nam, Y., Wheeler, B. C. In Vitro Microelectrode Array Technology and Neural Recordings. Crit Rev Biomed Eng. 39 (1), 45-62 (2011).
- Gonzalez-Sulser, A., et al. Hippocampal neuron firing and local field potentials in the in vitro 4-aminopyridine epilepsy model. J Neurophysiol. 108 (9), 2568-2580 (2012).

