Testing for Odor Discrimination and Habituation in Mice
Summary
This manuscript describes a protocol to examine the olfactory system of rodents. The olfactory habituation/dishabituation test will allow investigators to determine whether a mouse habituates to a repeatedly presented odor and whether the mouse demonstrates dishabituation when presented a novel odor.
Abstract
This video demonstrates a technique to establish the presence of a normally functioning olfactory system in a mouse. The test helps determine whether the mouse can discriminate between non-social odors and social odors, whether the mouse habituates to a repeatedly presented odor, and whether the mouse demonstrates dishabituation when presented with a novel odor. Since many social behavior tests measure the experimental animal’s response to a familiar or novel mouse, false positives can be avoided by establishing that the animals can detect and discriminate between social odors. There are similar considerations in learning tests such as fear conditioning that use odor to create a novel environment or olfactory cues as an associative stimulus. Deficits in the olfactory system would impair the ability to distinguish between contexts and to form an association with an olfactory cue during fear conditioning.
In the odor habitation/dishabituation test, the mouse is repeatedly presented with several odors. Each odor is presented three times for two minutes. The investigator records the sniffing time directed towards the odor as the measurement of olfactory responsiveness. A typical mouse shows a decrease in response to the odor over repeated presentations (habituation). The experimenter then presents a novel odor that elicits increased sniffing towards the new odor (dishabituation). After repeated presentation of the novel odor the animal again shows habituation. This protocol involves the presentation of water, two or more non-social odors, and two social odors. In addition to reducing experimental confounds, this test can provide information on the function of the olfactory systems of new knockout, knock-in, and conditional knockout mouse lines.
Introduction
Mice are dependent on olfaction for navigating new environments, finding food, for recognizing other individuals, and sexual behavior1-3. It is essential that investigators establish whether or not experimental animals have a functioning sense of smell before administering behavioral tests that involve food, social interaction, or any odors intended to elicit a response from the mouse. Anosmia or the inability to distinguish between different odors, could result in false positives or negatives in a wide variety of behavioral paradigms, so a mouse’s ability to detect and distinguish odors should be established before other types of behavioral tests are performed.
The olfactory habituation/dishabituation test was first described in the 1980s4. The test has been adapted for use in mice by Drs. Mu Yang and Jacqueline Crawley5. This is a simple and inexpensive test that allows the investigator to establish that a mouse can detect and differentiate between odors. In addition to testing olfaction, this test allows the investigator to observe the general behavior of the mouse and should be done early in a testing regimen. Qualitative observations regarding the mouse’s locomotion, signs of anxiety, level of activity, and response to social odors versus food odors may signal new areas in which testing could be carried out.
In this test, cotton swabs dipped into various odors are presented to a mouse three times in a row. With each repeated presentation of an odor, the mouse will habituate to the cotton swab, spending less time investigating it with each subsequent presentation. When a new odor is presented, dishabituation occurs, and a typical mouse will spend more time investigating the swab, indicating that it can discriminate between the current and previous odors5. This test is administered to one mouse at a time and includes a 45 min acclimation period followed by 45 min of testing.
Although this test is easy to carry out it may be used to investigate sophisticated questions about the mouse olfactory system. Other popular tests of olfaction, such as the buried food test, simply establish the presence or absence of anosmia. However, the olfactory discrimination and habituation test allows the investigator to determine that a mouse not only has the ability to detect odors but can discriminate between different odors. The pattern of habituation and dishabituation has been used to establish that new mutants can discriminate between odors6,7. In a surprising study, Fadool and colleagues used complex mixtures of odors to show that mice with gene-targeted deletion of the Kv1.3 channel are “super smellers” that can discriminate very similar odors better than normal mice8.
When examining a new knockout mouse model it is useful to establish the presence of normal behavior for basic sensory tasks. When done early in a testing regimen, the odor discrimination and habituation test gives an investigator the opportunity to observe any unusual behaviors. These observations could prevent false positive or negative results in subsequent testing that are attributable to confounding characteristics of the mutant. As researchers continue to investigate social behavior the need to verify basic olfactory function becomes increasingly important. In addition to examining olfaction in mutant lines, this test can be used to address specific questions such as whether a pharmacological treatment specifically increases an animal’s response to social odor stimuli or if their response increases to all odor stimuli.
Protocol
The adult male mice used in the following experiment were generated and housed at Baylor University at an ambient temperature of 22 °C, with a 14 hr light and 10 hrs dark (20:00 to 6:00 hr) diurnal cycle. The mice were given ad libitum access to food and water. All procedures to the mice were in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the animal protocol was approved by Baylor University Animal Care and Use Committee. Mice were tested in the late morning/early afternoon. For all procedures below the investigator should wear gloves to eliminate odors and prevent contamination.
1. Preparation of Non-social Odors
- Prepare fresh non-social odors each day of testing.
Note: The investigator should use gloves to prepare the odors.- Fill 3 centrifuge tubes with 10 ml of water each.
- Pipette 100 µl of banana extract into a 15 ml conical tube and 100 µl of almond extract into a second 15 ml conical tube for a 1:100 dilution.
Note: The third 15 ml tube will only contain water.
2. Preparation of Social Odors
- Prepare fresh social odors on the morning testing will occur. Obtain social odors from two cages that have not been cleaned for at least three days and contain equal numbers of mice. Obtain social odors from mice of the same sex as the test subject.
Note: These are to be stored at RT and should be used within 4-6 hr to maintain strong and consistent odor.- Determine how many social odor cotton swabs need to be created: each mouse being tested will be presented with three swabs from social odor 1, and three from social odor 2.
- Prepare social odors by wiping a cotton swab in a zigzag fashion across the bottom of a dirty cage. Shake off any excess bedding clinging to the tip.
- Store swabs in large glass jars with lids or in beakers covered with parafilm. Designate one container for social odor 1 and a second for social odor 2.
- Maintain odor source cages outside of the experimental testing room.
3. Acclimation of the Test Mouse
- Put on gloves and weigh the mouse to be tested and place individually in a clean cage with a wire lid on top and the water bottle removed.
- Allow the mouse to acclimate to the new cage for 45 min in a room other than the testing room. Ensure that there are no unusual odors or noises in the room.
- As testing takes about 45 min, stagger the acclimation times by putting the next mouse to be tested into a clean cage to acclimate right before testing the current mouse.
4. Preparation of the Testing Room
- Place fresh cotton swabs, laboratory tape, the bottles containing social odor swabs, the tubes of non-social odors, a micropipette, two timers, and a waste bottle with a lid in the testing room.
- Ensure that the testing room is reasonably free of odors, loud noises, and bright light. Do not wear scented colognes or lotions.
Note: The observer should not be chewing gum or candy during the testing period and should try to limit their exposure to strong odor foods.
5. Testing Procedure
- Bring the acclimated mouse in its cage into the testing room and place the cage on the counter at a height that is comfortable for viewing while seated.
- Present each odor 3 times in a row for 2 min each time. Use the presentation order that can be found in Table 1.
- Pipette 50 µl of odorant in the scent to be tested on to the tip of the cotton swab and re-cap the odor source. Lift the wire top of the cage and insert the unscented, wooden end of the swab into the water bottle opening, pushing the length through from the underside of the wire to the top. Avoid touching the cotton tip to anything, including fingers, so that odor contamination is avoided.
- Continue to draw the swab through the water bottle opening until about 1 inch of the cotton end hangs down into the cage. Affix the wooden end of the swab to the side of the cage using lab tape.
Note: Be careful not to let the lid fall back onto the cage, causing a loud noise that would disturb the test mouse. - Immediately begin timing for 2 min. Use one timer to time the 2 min presentation. Record the cumulative time spent sniffing the odor during the 2 min trial. Count the animal’s sniffing behavior using a separate stop watch. Active sniffing is defined when the animal’s nose is oriented towards the cotton tip with its nose ~2 cm or closer to the tip.
- After the completion of each trial, raise the wire lid and remove the cotton swab by pulling it downwards through the underside of the lid (this avoids odor contamination by preventing the cotton tip from touching the wire). Seal the used swab in a 500 ml waste bottle.
- Prepare and insert the next swab and begin timing. Allow approximately 1 min between each inter-trial interval. Continue until each scent has been presented three times in a row, for 2 min each time.
- Once the mouse has completed all trials, empty the waste bottle containing the used cotton swabs in a trash can outside of the test room. Return the mouse to its home cage.
6. Analysis
- Analyze data for each group per odor condition using two-way ANOVA followed by Newman-Keuls or a similar post hoc test to determine significant habituation (less time sniffing successive same smells), and dishabitutation (more time sniffing a new smell).
Representative Results
Following the protocol described, results were recorded using ten adult (postnatal day 90-120) male C57BL/6J mice and eight male FVB mixed-background strain (postnatal day 50-70). Testing was carried out by a single investigator over multiple days. Each point on the graphs represents the average time spent investigating an odor.
When the cotton swab dipped in water is first introduced, the mice spend a great deal of time investigating this novel object (Figure 1: first three data points). With each successive presentation, less time is spent investigating the swab, indicating habituation. When a new odor is introduced, there is an uptick in time spent investigating the swab, indicating that the mice can discriminate between the new odor and the previous one (Figure 1: Almond data points). Again, with each successive presentation, less time is spent investigating the odor. The mice show a significant decrease in time sniffing the swab of water over the three trials F(2,18) = 3.8, p <0.05. They show a similar pattern when repeatedly presented with the almond odor F(2,18) = 3.6, p <0.05. By the third odor they are still demonstrating habituation but by this trial the animals are reaching a floor effect (Figure 1: Banana data points). The floor effect is when the behavior is so low that the ability to measure the behavior any lower is not reliable. They do not show a change over the three trials when presented with the banana odor F(2,18) = 2.5, p <0.1. However, a two-way ANOVA for all 9 presentations of odor show a significant effect over time F(8,72) = 3.8, p <0.001.
The same pattern of habituation and discrimination can be seen with social odors as with the non-social odors. However, the mice spend more time investigating the social odors (Figure 2). There is a significant decrease in time spent sniffing the social odors when all 6 trials were combined F(2,18) = 6.1, p <0.01. However, there was a marginal effect over the first three trials F(2,18) = 3.0, p = 0.076. This suggests there was some habituation but not a statistically significant amount of reduction in time sniffing the first social odor. There was a significant reduction in the amount of time the animal investigated the social odor F(2,18) = 6.1, p <0.01 during the second presentation of a social odor.
We repeated the method in a male FVB mixed-background strain. When the cotton swab dipped in water is first introduced, the mice spend a great deal of time investigating this novel object (Figure 3: first three data points). With each successive presentation, less time is spent investigating the swab, indicating habituation. When a new odor is introduced, there is an uptick in time spent investigating the swab, indicating that the mice can discriminate between the new odor and the previous one (Figure 3: Almond data points). Again, with each successive presentation, less time is spent investigating the odor. The mice show a significant decrease in time sniffing the swab of water over the three trials F(2,14) = 13.3, p <0.001. They show a similar pattern when repeatedly presented with the almond odor F(2, 14) = 12.7, p <0.001. In this strain there was still a significant difference across the three trials. By the third odor they are still demonstrating habituation (Figure 3: Banana data points) but due to the floor effect they do not show a change over the three trials when presented with the banana odor F(2,14) = 3.04, p = 0.08. However, a two-way ANOVA for all 9 presentations of odor show a significant effect over time F(8,56) = 9.6, p <0.001.
The same pattern of habituation and discrimination was not seen with social odors as with the non-social odors. There was not a significant decrease in time spent sniffing the social odors when all 6 trials were combined F(5,35) = 0.16, p = 0.97. The data suggest that the mice do not habituate to repeated presentations of two social odors.
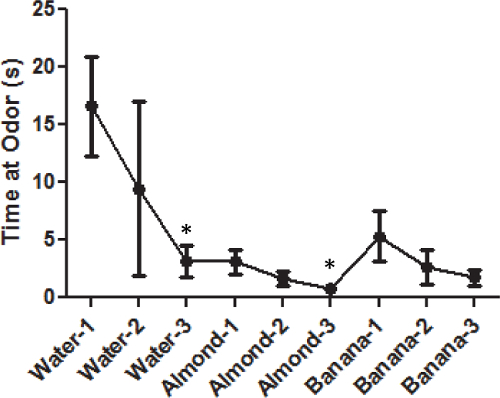
Figure 1: Average time spent investigating non-social odors for male C57BL/6J mice. Each data point represents the mean time the animal spent investigating the particular non-social odor. The error bars represent the standard error of the mean. Asterisks (*) indicate a significant difference where the group is displaying habituation (p <0.05).
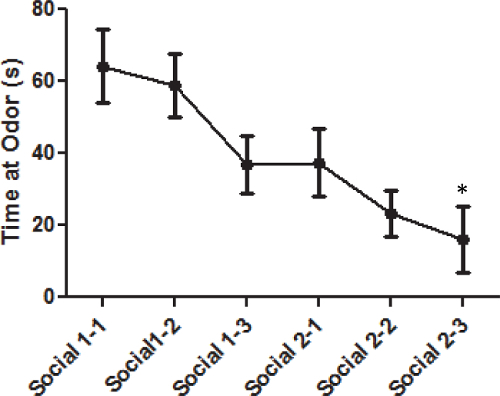
Figure 2: Average time spent investigating social odors for male C57BL/6J mice. Each data point represents the mean time the animal spent investigating the particular social odor. The error bars represent the standard error of the mean. Asterisks (*) indicate a significant difference where the group is displaying habituation (p <0.05).
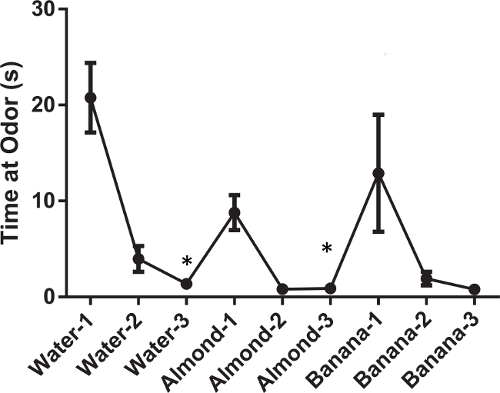
Figure 3: Average time spent investigating non-social odors for male FVB mixed-background strain. Each data point represents the mean time the animal spent investigating the particular non-social odor. The error bars represent the standard error of the mean. Asterisks (*) indicate a significant difference where the group is displaying habituation (p <0.05).
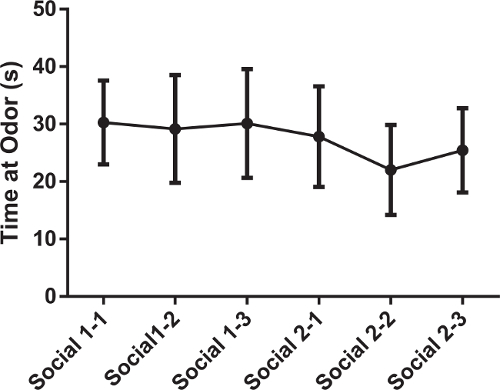
Figure 4: Average time spent investigating social odors for male FVB mixed-background strain. Each data point represents the mean time the animal spent investigating the particular social odor. The error bars represent the standard error of the mean.
| Odor |
| Water |
| Water |
| Water |
| Almond |
| Almond |
| Almond |
| Banana |
| Banana |
| Banana |
| Unfamiliar social odor 1 |
| Unfamiliar social odor 1 |
| Unfamiliar social odor 1 |
| Unfamiliar social odor 2 |
| Unfamiliar social odor 2 |
| Unfamiliar social odor 2 |
Table 1
Discussion
The results provided in this paper are optimal for mice. The mice demonstrate a strong response to a novel odor, then quickly habituate. One of the key steps in this method is the preparation of the odors. The investigator needs to take great care to isolate the odors from one another to prevent contamination. Another key aspect is the presentation of the cotton swab. The investigator needs to troubleshoot the best location of the cotton tip so the mouse can investigate the tip but does not tear at the tip. An important component of the test is the selection of odors. We presented three non-social odors but it would not be difficult to include additional odors. One caveat is to choose odors that are noticeably different from one another. One may observe a “floor effect” if similar odors are presented sequentially. Additionally, the mice may begin to reduce their response when presented with too many novel odors. If the investigator wants to include additional odors then he/she may want to include additional days of testing. It takes approximately one hour to test each mouse. Furthermore, one should troubleshoot the selection of odors in control mice before including them as an experimental condition.
The olfactory habituation/dishabituation is an important test to include when investigating social behavior. The three chamber social behavior test is commonly used to examine social behavior deficits in mice. This test includes a condition where the experimental animal investigates a novel object and a novel mouse which are placed under a cup at different ends of the chamber. The test can also include a condition where a novel mouse is placed under one cup and another mouse (familiar mouse) is placed under the other cup. The investigator then examines whether the experimental mouse can differentiate between the two. Since olfaction is the main sensory modality mice use to identify other mice9-11, impaired olfaction could impair their ability on this task. Impaired olfaction could result in misinterpretation of data. For instance, the experimenter may observe that the mouse does not spend more time with the novel mouse compared to the familiar mouse. This may appear to demonstrate evidence of social deficits or impaired social memory in the mice being tested. However, a simpler interpretation may be that the animal cannot properly detect the animal.
The olfactory habituation/dishabituation test has been useful in clarifying mouse models of autism. One often used mouse model of autism is the BTBR T + tf/J (BTBR) mouse strain. This is an inbred strain that has strong face validity to the three core behavioral characteristics of autism. However, one concern has been whether their social behavior deficits are due to an impaired olfactory system. Yang and colleagues investigated whether the BTBR strain has impaired olfaction through the use of the habituation/dishabituation test12. They used three presentations of water, almond odor, and banana odor for their nonsocial odors. They then used a social odor prepared from 129/SvlmJ and B6 mouse strains. The BTBR strain displayed habituation and dishabituation to the nonsocial odors. The mice demonstrated habitation to the first social odor but did not show dishabituation to the second social odor. One possibility is that they may not be able to differentiate between different social odors. This could impair their ability in a social behavior test when two social stimuli are presented. This is an important consideration when exploring different mouse models of autism and could provide new insights into what is altered in these mice.
Habituation and dishabituation are basic non-associative mechanisms of learning. These learning principles have been demonstrated in numerous types of complex and simple organisms13,14. However, these mechanisms of learning have been investigated infrequently in mouse olfactory behavior but this system is important to measure as part of a larger battery of behavioral tests. This test is inexpensive to setup and run, requires only one day of testing, and several mice can be tested within one day. The main objective of this test is to examine olfactory function. The results from the test can help reduce misinterpretation of social behavior tests and other tests which include an odor component.
When examining mouse behavior in any task there are several considerations. The strain of mouse, the sex of the animals, and time of day are all factors that need to be examined. If the investigator is using a mixed strain then there may be more variability in the investigation behavior of odors. We presented data from a mixed FVB strain. They performed well in the non-social odor tests (Figure 3). Although, they investigated the social odor for a high amount of time, they did not demonstrate habituation to repeated presentations of social odors (Figure 4). The mice may need to undergo more trials to demonstrate habituation. We have provided a general method that should be useful for most strains. However, the factors above should be considered when examining the behavior of any strain for the first time. By examining the performance of the control animals the investigator may need to adjust the odors selected, the number of times the odor is presented, the lighting conditions, time of day for testing, and the sex of the animal. This is true for any other mouse behavior and should be considered in this type of testing. We have included odors that are easily available for purchase. If the investigator is concerned that they are not using pure odors then they may consider ordering from Sigma-Aldrich. They have a database of over 1,600 aroma raw materials. This will allow the investigator to use a pure odor but the cost may be prohibitive to some labs.
There are some testing conditions the experimenter may want to consider. In many tests the animal is acclimated to the room it will be tested in. In this protocol we acclimated the experimental animal in one room and tested it in the other. This will cause some arousal when moved to the new room. We performed the procedure this way because if all of the mice are housed in the same room as the testing mice then all mice will be exposed to the novel odors. There are two options that the experimenter may want to consider. The first is what we described above. The other is to move the animal to the room to let it acclimate to the room then start testing. After testing is complete then have the next animal placed in the room then allowed to acclimate. This is possible but would nearly double the time of the experiment. The option we used was to move the testing animal to the testing room only during the experiment. This would result in less testing time. There is a balance in testing efficiency and concern about novelty induced arousal. The experimenter should determine which is best for their experiment.
Wherein this protocol, a person scores the behavior real-time, it has become increasingly popular to study animal behavior with cameras to record their behavior. There are several advantages to the use of video recordings of animal behavior. One is that a human investigator in the room may influence the mouse behavior. Indeed, one study found that having a male experimenter can influence the pain sensitivity of mice being tested15. Another concern is that the results are only from one investigator. Through the use of video recordings multiple observers can score behavior. One can then measure how reliable the scoring is between observers, which will result in a more consistent measurements. One other advantage is that the frame speed of the videos can be reduced and may provide a more accurate measurement of the behavior. The use of cameras can easily be introduced into this method. The setup will depend on the lab space configuration. The disadvantage of using cameras is that the scoring time will significantly increase. Requiring two or more observers to score the behavior will increase the time needed to produce the results. This will increase the labor burden to the lab and in some situations may be prohibitive if screening many knockout lines. Another disadvantage is that more than one camera may be needed. The camera will be in a fixed position. If the mouse investigates the odor in a way that prohibits the observer to see the animal then the trial may need to be eliminated. This is less of a concern with an individual who can easily adjust their view of the animal by adjusting their line of sight. However, the method described in this protocol can easily be adjusted to include video recordings without significant alteration to the protocol we describe.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by a Baylor University Research Council grant and by a research grant from the Epilepsy Foundation.
Materials
| Mouse cage | Allentown | Standard mouse cage | |
| Wire lid | Allentown | BCU Mouse WBL 2500 | |
| Bedding | Harlan | 7090 Sani-Chips | |
| Cotton swabs | VWR | 89031-270 | 6” wooden handle |
| Banana extract | McCormick | ||
| Almond extract | McCormick | ||
| Laboratory tape | VWR | 89098-062 | |
| Stop watch | VWR | 62374-000 | |
| Nitrile gloves | VWR | 82026 | |
| Timing device | VWR | 61161-350 | |
| 15 mL conical tubes | VWR | 89003-294 | |
| 2 L beakers | Pyrex | 1003 | |
| Parafilm | Parafilm | PM-992 | 4” x 250’ |
| 1 L bottle with cap | VWR | 89000-240 |
References
- Doty, R. L. Odor-guided behavior in mammals. Experientia. 42, 257-271 (1986).
- Restrepo, D., Arellano, J., Oliva, A. M., Schaefer, M. L., Lin, W. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 46, 247-256 (2004).
- Keverne, E. B. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 83, 177-187 (2004).
- Gregg, B., Thiessen, D. D. A simple method of olfactory discrimination of urines for the Mongolian gerbil, Meriones unguiculatus. Physiol Behav. 26, 1133-1136 (1981).
- Yang, M., Crawley, J. N. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. Chapter 8 (Unit 8 24), (2009).
- Pan, Y. W., Kuo, C. T., Storm, D. R., Xia, Z. Inducible and targeted deletion of the ERK5 MAP kinase in adult neurogenic regions impairs adult neurogenesis in the olfactory bulb and several forms of olfactory behavior. PLoS ONE. 7, e49622 (2012).
- Wesson, D. W., Levy, E., Nixon, R. A., Wilson, D. A. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer’s disease mouse model. J Neurosci. 30, 505-514 (2010).
- Fadool, D. A., et al. Kv1.3 channel gene-targeted deletion produces ‘Super-Smeller Mice’ with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 41, 389-404 (2004).
- Arakawa, H., Arakawa, K., Blanchard, D. C., Blanchard, R. J. Scent marking behavior in male C57BL/6J mice: sexual and developmental determination. Behav Brain Res. 182, 73-79 (2007).
- Baum, M. J. Sexual differentiation of pheromone processing: links to male-typical mating behavior and partner preference. Horm Behav. 55, 579-588 (2009).
- Ferguson, J. N., et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 25, 284-288 (2000).
- Yang, M., et al. Low sociability in BTBR T+tf/J mice is independent of partner strain. Physiol Behav. 107, 649-662 (2012).
- Best, J. D., et al. Non-associative learning in larval zebrafish. Neuropsychopharmacology. 33, 1206-1215 (2008).
- Wolman, M. A., Jain, R. A., Liss, L., Granato, M. Chemical modulation of memory formation in larval zebrafish. Proc Natl Acad Sci U S A. , (2011).
- Sorge, R. E., et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 11, 629-632 (2014).

