Culture of Macrophage Colony-stimulating Factor Differentiated Human Monocyte-derived Macrophages
Summary
A protocol is presented for cell culture of macrophage colony-stimulating factor (M-CSF) differentiated human monocyte-derived macrophages. The protocol utilizes cryopreservation of monocytes coupled with their bulk differentiation into macrophages. Then harvested macrophages can then be seeded into culture wells at required cell densities for carrying out experiments.
Abstract
A protocol is presented for cell culture of macrophage colony-stimulating factor (M-CSF) differentiated human monocyte-derived macrophages. For initiation of experiments, fresh or frozen monocytes are cultured in flasks for 1 week with M-CSF to induce their differentiation into macrophages. Then, the macrophages can be harvested and seeded into culture wells at required cell densities for carrying out experiments. The use of defined numbers of macrophages rather than defined numbers of monocytes to initiate macrophage cultures for experiments yields macrophage cultures in which the desired cell density can be more consistently attained. Use of cryopreserved monocytes reduces dependency on donor availability and produces more homogeneous macrophage cultures.
Introduction
Study of cultured macrophages is a useful model to understand the function of these cells in inflammation such as occurs in atherosclerotic plaques. When the research focus is on human diseases involving macrophages, it is useful to study primary human macrophages rather than non-human macrophages to avoid species differences. Also, the effects of cell transformation can be avoided by using primary macrophages rather than macrophage cell lines. For this purpose, macrophages differentiated from monocytes isolated from human blood serve as a means of obtaining primary human macrophages.
Tissue macrophages may be either resident within tissues or may be derived from monocytes that migrate into the tissue and differentiate into macrophages 1. Two types of human monocyte-derived macrophages have been defined that differ not only in morphology but also gene expression and cell function 2. These two types are obtained from monocytes that are differentiated into macrophages in the presence of M-CSF + fetal bovine serum (FBS) and monocytes that are differentiated into macrophages in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) + FBS 2-6. In our experience (unpublished observation), use of human serum rather than FBS generates the GM-CSF type regardless of whether M-CSF or GM-CSF is included in the differentiation medium. M-CSF type macrophages tend to be more elongated than GM-CSF type macrophages, which resemble fried eggs in their morphology. Investigators should recognize that human M-CSF and GM-CSF monocyte-derived macrophages are not the same as so called M1 and M2 mouse bone marrow-derived macrophages 6.
During research using human monocyte-derived M-CSF differentiated macrophages, we experienced difficulty related to the availability of monocytes to initiate experiments and variation in the obtained cell densities of macrophages during differentiation of monocytes into macrophages. To overcome these problems, we have developed the following protocol in which monocytes are frozen until required for use, and monocytes are differentiated in bulk into macrophages that can then be removed from culture flasks and plated at desired cell densities to obtain more uniform cultures from experiment to experiment.
Protocol
Leukapheresis was carried out under a human subject's research protocol approved by a National Institutes of Health institutional review board.
1. Isolation and Cryopreservation of Monocytes
- Obtain mononuclear cells by leukapheresis of human donors, and enrich monocytes by continuous counter-flow centrifugation elutriation of mononuclear cells as described in the references 7,8. Obtain approximately 100 x 106 elutriated cells (approximately 80 – 90% monocytes) in elutriation buffer specified by the manufacturer. Count cells using a hemocytometer.
- Centrifuge monocytes in a 15 ml polypropylene tube at 300 x g for 5 min at room temperature.
- Remove the supernatant and gently resuspend the cells in FBS followed by dimethyl sulfoxide to achieve a final concentration of 90% FBS/10% dimethyl sulfoxide and 50 x 106 cells/ml.
- Add each ml of cell suspension to an individual cryovial.
- Place the cryovials in a cell freezing container and transfer to a -80 °C freezer for 24 hr before transferring to a liquid nitrogen cryovial storage tank for long-term storage.
2. Differentiation of Monocytes into Macrophages
- Thaw a cryovial of cells by quickly transferring to a 37 °C water bath and then immediately removing when the cell suspension has thawed about 70%.
- Immediately upon complete thawing at room temperature, transfer the 1 ml cryovial contents into 50 ml of 37 °C warmed Roswell Park Memorial Institute (RPMI) 1640 medium with 2 mM L-glutamine, 50 ng/ml M-CSF, 25 ng/ml interleukin-10 (IL-10), and 10% FBS (complete medium).
- Transfer 25 ml of monocyte suspension into each of two 75 cm2 plastic cell culture flasks.
- Incubate cultures in a 37 °C cell culture incubator with 5% CO2 / 95% air for 48 hr.
- Rinse the cultures 3 times with 10 ml RPMI 1640 medium (pre-warmed to 37 °C), gently removing the culture medium to avoid dislodging any loosely attached cells.
- Following rinsing, add fresh complete medium, changing medium every 2 days until monocytes differentiate and proliferate sufficiently to become confluent. This requires about one week of culture.
3. Harvesting Macrophages to Initiate Experiments
- Rinse differentiated macrophages in flask 3 times with 10 ml pre-warmed 37 °C Dulbecco's phosphate-buffered saline without Ca2+ and Mg2+ before adding 10 ml pre-warmed 37 °C 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA) solution.
- Place flask in cell culture incubator for 10 – 15 min at which time approximately 90% of the macrophages should have rounded and detached. Verify this by microscopic examination of the flask.
- Add 10 ml of RMPI 1640 medium containing 10% FBS to stop trypsinization.
- Transfer the macrophage cell suspension from the flask into a 50 ml polypropylene tube, centrifuge 300 x g for 5 min, and discard the supernatant.
- Gently resuspend the macrophages into 1 ml of complete medium.
- Mix 10 µl of macrophage cell suspension with 10 µl Trypan Blue solution and determine macrophage cell number and viability (typically > 95%) using a hemocytometer.
- Based on the cell count (usually about 15 – 18 x 105 macrophages), add additional complete medium to achieve the desired seeding cell density. Note: 1 x 105 macrophages in 1.5 ml medium per well of a 12-well plate will produce a near-confluent culture.
- Following seeding, incubate macrophages overnight in a 37 °C cell culture incubator with 5% CO2 / 95% air to allow cell attachment. Then, begin desired experiments.
Representative Results
The viability of fresh or cryopreserved monocytes was greater than 95% as determined with Trypan Blue staining 9. Figure 1 and Figure 2 compare at lower and higher magnifications, respectively, the progress of fresh and cryopreserved (i.e., frozen) monocyte differentiation into macrophages. Note that the fresh compared with cryopreserved monocytes show a subpopulation of differentiating monocytes that do not spread but remain rounded. Phase-lucent vacuoles occur in the monocyte-derived macrophages in both conditions. These vacuoles represent macropinosomes characteristic of M-CSF type macrophages as previously described 10,11.
Cryopreserving macrophages after their harvesting from the flasks in which they were differentiated from monocytes did not produce successful macrophage cultures (Figure 3). Such cultures showed very sparse cell attachment possibly due to poor viability after cryopreservation.
The above protocol for producing M-CSF type macrophages does so without the presence of contaminating GM-CSF type macrophages that sometimes occurs when cultures are established directly from monocytes without their cryopreservation and differentiation to M-CSF type macrophages in flasks. However, cryopreserved monocytes differentiated with FBS + GM-CSF produce excellent GM-CSF type cultures (Figure 4).
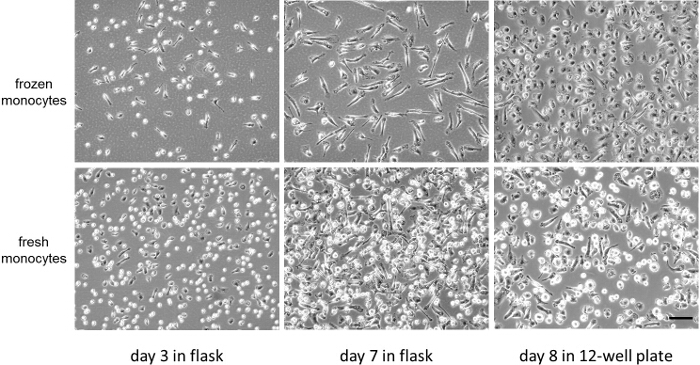
Figure 1: Comparison of fresh versus cryopreserved monocytes used to produce macrophages (low magnification). Phase-contrast microscopic images of cryopreserved (frozen) and fresh monocytes during their M-CSF induced differentiation into macrophages. Shown are monocytes during culture in flasks at 3 and 7 days, and on day 8, one day after their harvesting and replating into 12-well culture plates. Bar = 100 µm and applies to all. Please click here to view a larger version of this figure.
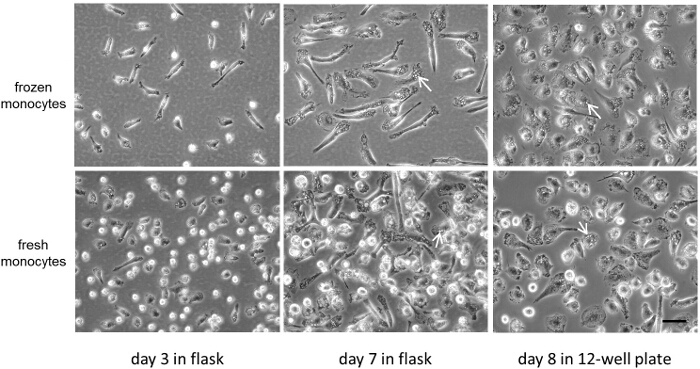
Figure 2: Comparison of fresh versus cryopreserved monocytes used to produce macrophages (high magnification). Monocytes were cultured as described in Figure 1 legend and shown here at higher magnification. Phase-lucent vacuoles are macropinosomes (white arrows). Bar = 50 µm and applies to all. Please click here to view a larger version of this figure.
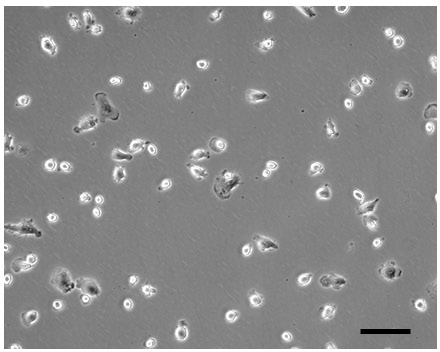
Figure 3: Cryopreserved macrophages fail to produce adequate cultures. Fresh monocytes were seeded into a flask and differentiated 7 days with M-CSF as shown in Figures 1 and 2 above. Then, the differentiated macrophages were harvested from flasks and cryopreserved. Next, the cryopreserved macrophages were seeded into 12-well plates as described in the protocol for non-cryopreserved macrophages and cultured 1 day (day 8). Phase-contrast microscopic image of the 1-day culture shows that very few of the macrophages attach following their cryopreservation (compare to day 8 results for non-cryopreserved macrophages in Figure 1 above). Bar = 100 µm. Please click here to view a larger version of this figure.
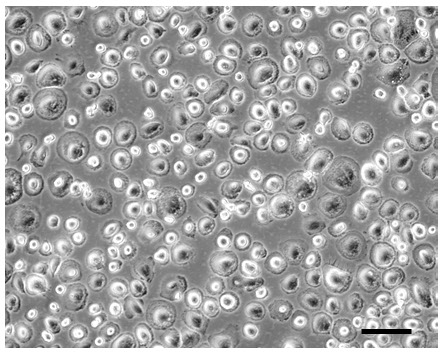
Figure 4: Cryopreserved monocytes were also suitable to produce GM-CSF type monocyte-derived macrophages. Cryopreserved monocytes (25 x 106) were seeded into a flask and differentiated 8 days with 10% FBS containing 50 ng/ml GM-CSF. Phase-contrast image shows the typical "fried egg" morphology of GM-CSF type macrophages. Bar = 100 µm. Please click here to view a larger version of this figure.
Discussion
Generating defined macrophage types can clarify some of the conflicting results obtained by investigators when studying macrophage biology. The use of various culture conditions and differentiation factors to generate primary human macrophages can lead to very different macrophage types, a fact that may not be appreciated by the researcher. For example, macrophages sometimes are generated from human monocytes using no serum, human serum alone, human serum supplemented with M-CSF, FBS alone, or FBS containing M-CSF or GM-CSF. As reported previously and as we have also observed, monocytes cultured for a week without serum or with FBS without added differentiation factors do not remain viable 3. As mentioned above, monocytes cultured with human serum alone or supplemented with M-CSF generate macrophages that show the "fried egg" morphology characteristic of monocytes differentiated with FBS plus GM-CSF. Thus, in the presence of human serum the M-CSF type macrophage showing a more elongated morphology is not obtained even though monocytes have been differentiated with M-CSF.
Interestingly, we have observed that differentiation of monocytes with FBS containing both M-CSF and GM-CSF, generates both the M-CSF elongated and GM-CSF "fried egg" morphological phenotypes in the same culture. This suggests that distinct monocyte subpopulations give rise to these two macrophage types. Previous studies have shown that M-CSF and GM-CSF differentiation factors affect not only the morphology of the macrophage, but also influence gene expression and cell function, which is substantially different for these two macrophage types 2-6. Immunostaining for the macrophage marker CD68 in conjunction with CD14 that is expressed by M-CSF type macrophages and 25F9 that is expressed by GM-CSF macrophages can be used to confirm the macrophage type as shown previously 2. These two macrophage types may be especially relevant to the study of atherosclerosis where different macrophage types occur in atherosclerotic plaques and can show differences in cholesterol metabolism and expression of inflammation mediators 2,12,13. For example, M-CSF type macrophages are unique in that these macrophages deposit cholesterol into the extracellular matrix when the macrophages are enriched with cholesterol 12,14,15.
In the protocol presented, during culture with M-CSF, IL-10 has been routinely added as described previously 16. This cytokine promotes the growth and differentiation of the macrophages and produces a more homogeneous elongated morphology of the macrophages. In addition, IL-10 inhibits GM-CSF-dependent monocyte survival by inhibiting the signaling events induced by GM-CSF 17. However, investigators should consider that IL-10 may affect other cell responses under study, and therefore, may wish to omit IL-10. Before using the current protocol employing proliferation and differentiation of the monocytes in a flask, macrophage cultures were initiated by directly plating elutriated monocytes into culture wells and differentiating them for 7 days prior to initiating experiments. However, even with the addition of IL-10, some of these macrophage cultures showed small numbers (usually less than 10%) of "fried egg" GM-CSF type macrophages contaminating the elongated M-CSF type macrophages. With the current protocol, GM-CSF type macrophage contamination does not occur for reasons that are not clear. However, it is not because GM-CSF macrophage type precursor monocytes are lost during freezing, as culturing the cryopreserved monocytes with FBS + GM-CSF produces excellent GM-CSF type cultures (see Figure 4).
The described protocol utilizes cryopreservation of monocytes before their differentiation into macrophages. Monocyte cryopreservation has been previously shown not to affect monocyte function and is commonly employed 18-21. In this regard, we have found that macrophages generated by the protocol above deposit cholesterol into the extracellular space similar to what we have described previously with non-cryopreserved monocytes that were cultured directly into macrophages 12. With monocyte cryopreservation, monocytes are always available to initiate experiments, independent of when the monocytes can be obtained from a donor. While it is not essential to cryopreserve the monocytes before proceeding with the protocol, cryopreservation did produce more homogeneous macrophage cultures in that all the macrophages were more spread rather than being both rounded and spread as was the case when fresh monocytes were used to initiate cultures. We have successfully generated macrophage cultures from monocytes that were kept frozen up to 6 months, (the longest time tested). On the other hand, seeding of cryopreserved macrophages into culture wells for experiments did not produce adequate macrophage cultures.
Critical steps in the protocol include immediately placing monocytes into the freezer following addition of dimethyl sulfoxide, and immediately transferring thawed monocytes to culture medium. Although it is common to remove DMSO by centrifuging frozen cells before they are seeded into culture, we did not find this was a necessary step in successful culture of the monocytes. However, investigators may need to do this if the concentration of residual DMSO (2 µl/ml) is higher than what was used in the above protocol. It is important not to rinse monocytes until 48 hours following their seeding in culture flasks. Otherwise, some monocytes may be lost depending on their degree of adhesion to the culture surface. On the other hand, once the monocytes differentiate into macrophages, increased adhesion of the macrophages may require longer trypsin treatment to remove them, or a cell lifter can be employed to facilitate release after the initial trypsin treatment. If monocytes do not proliferate and differentiate, this could be due to some unusual characteristic of the monocyte donor, loss of activity of M-CSF, or due to the lot of FBS used. Also, of possible concern when cultures do not develop is contamination of reagents with endotoxin, an agent that inhibits macrophage proliferation 22.
Differentiating the cryopreserved monocytes in flasks with M-CSF allows their bulk differentiation into macrophages. Then, the macrophages can be harvested and seeded into culture wells at required cell densities for carrying out experiments. The use of defined numbers of macrophages rather than defined numbers of monocytes to initiate macrophage cultures for experiments, yields macrophage cultures in which the desired cell density can be more consistently attained.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The Department of Transfusion Medicine, Clinical Center, National Institutes of Health, provided elutriated monocytes. This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, National Institutes of Health.
Materials
| Cellbind 12-well culture plate | Corning | 3336 | |
| CELLSTAR, T-75 flask, tissue culture treated | Greiner Bio-One North America | 658157 | |
| RPMI 1640 culture medium | Cellgro Mediatech | 15-040-CM | warmed to 37 °C |
| L-Glutamine | Cellgro Mediatech | 25-005-CI | |
| Fetal bovine serum | Gibco | 16000-036 | |
| M-CSF | PeproTech | 300-25 | |
| GM-CSF | PeproTech | 300-03 | |
| IL-10 | PeproTech | 200-10 | |
| DMSO | Sigma | D2650 | |
| Cryovial | Thermo Scientific | 375418 | |
| DPBS without Ca2+ and Mg2+ | Corning cellgro | 21-031-CV | |
| 0.25% Trypsin-EDTA | Gibco | 25200-056 | |
| 50 ml polypropylene conical tube | Falcon | 352070 | |
| Trypan Blue | Lonza | 17-942E | |
| Neubauer-improved bright light hemocytometer | Paul Marienfeld GmbH & Co. KG | 610031 | http://www.marienfeld-superior.com/index.php/counting-chambers/articles/counting-chambers.html |
| CoolCell LX cell freezing container | BioCision | BCS-405 | other freezing containers also should be adequate for this step |
| Liquid Nitrogen Storage System, CryoPlus 1 | Thermo Scientific | 7400 | any liquid nitrogen tank should be adequate |
References
- Dey, A., Allen, J., Hankey-Giblin, P. A. Ontogeny and polarization of macrophages in inflammation: blood monocytes versus tissue macrophages. Front. Immunol. 5, 683 (2014).
- Waldo, S. W., et al. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 172, 1112-1126 (2008).
- Akagawa, K. S. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Int. J. Hematol. 76, 27-34 (2002).
- Akagawa, K. S., et al. Functional heterogeneity of colony-stimulating factor-induced human monocyte-derived macrophages. Respirology. 11 Suppl, S32-S36 (2006).
- Fleetwood, A. J., Lawrence, T., Hamilton, J. A., Cook, A. D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178, 5245-5252 (2007).
- Lacey, D. C., et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J. Immunol. 188, 5752-5765 (2012).
- Strasser, E. F., Eckstein, R. Optimization of leukocyte collection and monocyte isolation for dendritic cell culture. Transfus. Med. Rev. 24, 130-139 (2010).
- Kim, S., et al. Monocyte enrichment from leukapheresis products by using the Elutra cell separator. Transfusion (Paris). 47, 2290-2296 (2007).
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. Appendix 3 (Appendix 3B), (2001).
- Anzinger, J. J., et al. Native low-density lipoprotein uptake by macrophage colony-stimulating factor-differentiated human macrophages is mediated by macropinocytosis and micropinocytosis. Arterioscler. Thromb. Vasc. Biol. 30, 2022-2031 (2010).
- Zhao, B., et al. Constitutive receptor-independent low density lipoprotein uptake and cholesterol accumulation by macrophages differentiated from human monocytes with macrophage-colony-stimulating factor (M-CSF). J. Biol. Chem. 281, 15757-15762 (2006).
- Freeman, S. R., et al. ABCG1-mediated generation of extracellular cholesterol microdomains. J. Lipid Res. 55, 115-127 (2014).
- Kruth, H. S. Receptor-independent fluid-phase pinocytosis mechanisms for induction of foam cell formation with native low-density lipoprotein particles. Curr. Opin. Lipidol. 22, 386-393 (2011).
- Jin, X., et al. ABCA1 contributes to macrophage deposition of extracellular cholesterol. J. Lipid Res. 56, 1720-1726 (2015).
- Ong, D. S., et al. Extracellular cholesterol-rich microdomains generated by human macrophages and their potential function in reverse cholesterol transport. J. Lipid Res. 51, 2303-2313 (2010).
- Hashimoto, S., Yamada, M., Motoyoshi, K., Akagawa, K. S. Enhancement of macrophage colony-stimulating factor-induced growth and differentiation of human monocytes by interleukin-10. Blood. 89, 315-321 (1997).
- Hashimoto, S. I., Komuro, I., Yamada, M., Akagawa, K. S. IL-10 inhibits granulocyte-macrophage colony-stimulating factor-dependent human monocyte survival at the early stage of the culture and inhibits the generation of macrophages. J. Immunol. 167, 3619-3625 (2001).
- Lund, P. K., Joo, G. B., Westvik, A. B., Ovstebo, R., Kierulf, P. Isolation of monocytes from whole blood by density gradient centrifugation and counter-current elutriation followed by cryopreservation: six years’ experience. Scand. J. Clin. Lab. Invest. 60, 357-365 (2000).
- Weiner, R. S., Normann, S. J. Functional integrity of cryopreserved human monocytes. J. Natl. Cancer Inst. 66, 255-260 (1981).
- Hansen, J. B., et al. Retention of phagocytic functions in cryopreserved human monocytes. J. Leukoc. Biol. 57, 235-241 (1995).
- Seager Danciger, J., et al. Method for large scale isolation, culture and cryopreservation of human monocytes suitable for chemotaxis, cellular adhesion assays, macrophage and dendritic cell differentiation. J. Immunol. Methods. 288, 123-134 (2004).
- Jin, X., Xu, Q., Champion, K., Kruth, H. S. Endotoxin contamination of apolipoprotein A-I: effect on macrophage proliferation–a cautionary tale. Atherosclerosis. 240, 121-124 (2015).

