Extraction of Structural Extracellular Polymeric Substances from Aerobic Granular Sludge
Summary
The protocol provides a methodology to solubilize aerobic granular sludge in order to extract alginate-like extracellular polymers (ALE).
Abstract
To evaluate and develop methodologies for the extraction of gel-forming extracellular polymeric substances (EPS), EPS from aerobic granular sludge (AGS) was extracted using six different methods (centrifugation, sonication, ethylenediaminetetraacetic acid (EDTA), formamide with sodium hydroxide (NaOH), formaldehyde with NaOH and sodium carbonate (Na2CO3) with heat and constant mixing). AGS was collected from a pilot wastewater treatment reactor. The ionic gel-forming property of the extracted EPS of the six different extraction methods was tested with calcium ions (Ca2+). From the six extraction methods used, only the Na2CO3 extraction could solubilize the hydrogel matrix of AGS. The alginate-like extracellular polymers (ALE) recovered with this method formed ionic gel beads with Ca2+. The Ca2+-ALE beads were stable in EDTA, formamide with NaOH and formaldehyde with NaOH, indicating that ALE are one part of the structural polymers in EPS. It is recommended to use an extraction method that combines physical and chemical treatment to solubilize AGS and extract structural EPS.
Introduction
In recent years the aerobic granular sludge (AGS) process has become a popular biological wastewater treatment process, successfully applied at several full-scale wastewater treatment plants1. In contrast to the conventional activated sludge process, in the AGS process the microorganisms form granules instead of flocs2. These granules have better settleability, are able to withstand higher organic loading rates, and have higher tolerance to toxicity than activated sludge flocs3.
Unlike biofilms, AGS is formed spontaneously without involvement of any carrier material4. In AGS, like in biofilms, microorganisms produce a significant amount of highly hydrated extracellular polymeric substances (EPS)5 to form a hydrogel matrix in which they are self-immobilized4–6. EPS are a complex mixture, consisting of polysaccharides, proteins, nucleic acids, (phospho)lipids, humic substances and some intercellular polymers5,7,8. These polymeric substances interact with each other through electrostatic forces, hydrogen bonds, attractive ionic forces and/or biochemical reactions, etc.5, forming a dense and compact tertiary network structure. The polymers in EPS which are able to form hydrogels4,9 and contribute to the formation of the tertiary network structure are in this respect considered as structural EPS, a subset of the total EPS.
EPS are responsible for the chemical structure and physical properties of granules5. It is therefore crucial to understand the function of each EPS compound. Various approaches are applied to extract EPS10–15. However, due to their extreme complexity, it is almost impossible to extract all the EPS components by one single method. To date, there is no "one size fits all" method for EPS extraction. The choice of the extraction method influences not only the total amount, but also the composition of the recovered polymers13,16–20. Depending on the type of sludge and the EPS of interest different methods are required.
Extracting gel-forming polymers, characterizing their properties and investigating their interactions with each other and with non-gel-forming EPS will help to reveal the role of EPS in aerobic granular sludge formation. Furthermore, the gel-forming polymers are also useful biopolymers in industrial applications. One possible application was already shown by using gel-forming polymers from AGS as a coating material to increase the water resistance of paper21.
Therefore, extraction methods, specific for gel-forming EPS are needed. The aim of this study is to develop a methodology to extract gel-forming EPS from AGS. Six extraction methods10–15,22, which are frequently used in literature, were selected to extract EPS from AGS. The total amount and the gel-forming property of the extracted EPS were compared for each methodology.
Protocol
NOTE: AGS was collected from the Nereda pilot reactor at the wastewater treatment plant Utrecht, the Netherlands. The reactor was fed with municipal sewage. The granular sludge had a sludge volume index (SVI5min) of 59.5 ml/gVSS. The sludge was sampled in April at the end of an aerobic cycle. After sampling, the sludge was immediately transported to the laboratory, sieved and stored at -20 °C until use.
1. EPS Extraction
NOTE: Centrifuge granular sludge at 4,000 × g and 4 °C for 20 min, and decant the supernatant. Collect granules in the pellet for the extractions. The total solids (TS) and volatile solids (VS) of the granules were determined by the standard methods23. The conversion factor between granule wet weight — the weight of the granules taken directly from the pellet — and the TS was determined prior to the extraction. All extractions were done in triplicates.
NOTE: 3 g wet granules were used for each extraction method. Their TS and VS values (0.39 g TS and 0.34 g VS), measured in triplicates, were used to calculate the extraction yield.
- Centrifugation extraction11
- Transfer 3 g (wet weight) of granules into a centrifugation tube and fill up the centrifugation tube to 50 ml with demineralized water.
- Slightly shake the centrifugation tube by hand.
- Centrifuge the centrifugation tube containing the mixture at 4,000 × g and 4 °C for 20 min.
- Collect the supernatant in a glass beaker, discard the pellet and continue with the supernatant as described in section 1.7.
- Sonication extraction10
- Transfer 3 g (wet weight) of granules into a centrifugation tube and fill up the centrifugation tube to 50 ml with demineralized water.
- Apply pulsed sonication on ice for 2.5 min at 40 W to the mixture.
- Centrifuge the centrifugation tube containing the mixture at 4,000 × g and 4 °C for 20 min.
- Collect the supernatant in a glass beaker, discard the pellet and continue with the supernatant as described in section 1.7.
- Ethylenediaminetetraacetic acid (EDTA) extraction11
- Transfer 3 g (wet weight) of granules into a 100 ml glass bottle and fill up the bottle to 50 ml with 2% (w/v) EDTA solution.
- Slightly shake the bottle by hand and store it in the refrigerator at 4 °C for 3 hr.
- Transfer the mixture into a 50 ml centrifugation tube.
- Centrifuge the centrifugation tube containing the mixture at 4,000 × g and 4 °C for 20 min.
- Collect the supernatant in a glass beaker, discard the pellet and continue with the supernatant as described in section 1.7.
- Formamide – sodium hydroxide extraction (NaOH)13
- Transfer 3 g (wet weight) of granules into a 100 ml glass bottle and fill up the bottle to 50 ml with demineralized water.
- Add 0.3 ml 99% formamide.
- Slightly shake the bottle by hand and store it in the refrigerator at 4 °C for 1 hr.
- Add 20 ml 1 M NaOH to the granule suspension.
- Slightly shake the bottle by hand and store it in the refrigerator at 4 °C for 3 hr.
- Transfer the mixture evenly into two 50 ml centrifugation tube.
- Centrifuge the centrifugation tubes containing the mixture at 4,000 × g and 4 °C for 20 min.
- Collect the supernatant in a glass beaker, discard the pellet and continue with the supernatant as described in section 1.7.
- Formaldehyde – NaOH extraction11
- Transfer 3 g (wet weight) of granules into a 100 ml glass bottle and fill up the bottle to 50 ml with demineralized water.
- Add 0.3 ml 37% formaldehyde.
- Slightly shake the bottle by hand and store it in the refrigerator at 4 °C for 1 hr.
- Add 20 ml 1 M NaOH to the granule suspension.
- Slightly shake the bottle by hand and store it in the refrigerator at 4 °C for 3 hr.
- Transfer the mixture evenly into two 50 ml centrifugation tube.
- Centrifuge the centrifugation tubes containing the mixture at 4,000 × g and 4 °C for 20 min.
- Collect the supernatant in a glass beaker, discard the pellet and continue with the supernatant as described in section 1.7.
- High temperature – sodium carbonate extraction (Na2CO3)9,22,24
- Pre-heat 150 ml tap water in a 1,000 ml glass beaker on a magnetic stirrer to 80 °C.
- Transfer 3 g (wet weight) of granules in a 250 ml baffled flask and fill up the flask to 50 ml with demineralized water.
- Add 0.25 g Na2CO3 anhydrous or 0.67 g Na2CO3•10H2O into the flask to obtain a 0.5% (w/v) Na2CO3 concentration.
- Put the flask containing the mixture into the water bath. Cover the flask and the beaker glass separately with aluminum foil to prevent evaporation.
- Stir the mixture for 35 min at 400 rpm and 80 °C.
- Transfer the mixture into a 50 ml centrifugation tube.
- Centrifuge the centrifugation tube containing the mixture at 4,000 × g and 4 °C for 20 min.
- Collect the supernatant and discard the pellet.
- TS and VS measurement of all extracts according to the standard methods23.
- Take the supernatant and dialyze it for 24 hr against 1,000 ml ultrapure water (dialysis bag with 3,500 Da molecular weight cut off (MWCO))11,12. Change the dialysis water after 12 hr to enhance the effect of the dialysis.
- Transfer a reasonable fraction (around 1/3) of the dialyzed supernatant to an aluminum dish for TS and VS measurement23.
NOTE: Dry the sample overnight at 105 °C. The weight difference of the empty aluminum dish and the aluminum dish containing the dried sample is the TS content. Then burn the same aluminum dish containing the sample at 550 °C for 2 hr. The weight difference between the empty aluminum dish and the aluminum dish containing the burned sample is the ash content. The difference between TS and ash content is the VS content. - For each extract, transfer the residual fraction of the dialyzed supernatant to 10 ml glass beakers. Thicken the supernatant for 2 days at 60 °C to a final volume of 1-2 ml to increase the polymer concentration in the supernatant.
2. Alginate-like Extracellular Polymer (ALE) Extraction
- Dialyze the extract obtained in step 1.6.8 according to step 1.7.1.
- Transfer the dialyzed extract into a 250 ml glass beaker. Slowly stir the extraction at 100 rpm and room temperature. Constantly monitor pH changes with a pH electrode, while adding 1 M hydrochloric acid (HCl) to a final pH of 2.2 ± 0.05 to obtain ALE in the acidic form.
- After adjusting the pH to 2.2, transfer the extract into a 50 ml centrifugation tube and centrifuge at 4,000 × g and 4 °C for 20 min.
- Discard the supernatant and collect the gel-like pellet. The gel-like pellet is ALE in the acidic form.
- To obtain the sodium (or potassium) form of ALE, slowly add 0.5 M NaOH (or 0.5 M potassium hydroxide) to the gel obtained in step 2.4, while mixing the gel slowly with a glass stick by hand until pH 8.5 is reached.
3. Ionic Hydrogel Formation Test
NOTE: In order to check if the extracted EPS had ionic hydrogel formation properties, a bead formation test with Ca2+ ions was used25.
- After thickening of the extract in step 1.7.3 to a volume of 1-2 ml, slowly stir the mixture with a glass stick and adjust its pH to 8.5 with 0.5 M NaOH.
- Take the extract of step 3.1 or the sodium ALE of step 2.5 and slowly drip the extract with a Pasteur pipette into a 2.5% (w/v) calcium chloride (CaCl2) solution.
NOTE: If the extracted EPS has ionic hydrogel gel forming properties, drop-shaped (spherical) beads will be formed. If the extracted EPS has no ionic hydrogel gel forming properties, the extract will disperse in the CaCl2 solution.
4. Stability Test of the Ionic Hydrogel
NOTE: To further understand the role of the ionic EPS hydrogel in AGS structure formation, stability tests were performed on the ionic hydrogel beads of the Na2CO3 extraction, collected in step 3.2.
- Keep the hydrogel beads for 30 min in the CaCl2 solution.
- Use a spoon to take out the hydrogel beads from the CaCl2 solution and split the beads in four equal fractions.
- Store fraction 1 in 10 ml demineralized water for 4 hr at 4 °C.
The following stability tests were performed in the same manner as described in the extraction methods 1.3 – 1.5. - Store fraction 2 in 10 ml 2% (w/v) EDTA solution for 3 hr at 4 °C.
- Store fraction 3 in 7.15 ml demineralized water with 60 µl 99% formamide for 1 hr at 4 °C. Then add 2.85 ml 1 M NaOH and store fraction 3 for 3 hr at 4 °C.
- Store fraction 4 in 7.15 ml demineralized water with 60 µl 37% formaldehyde for 1 hr at 4 °C. Then add 2.85 ml 1 M NaOH and store fraction 4 for 3 hr at 4 °C.
- Monitor if there is visible disintegration of the beads during the storage under the conditions described in 4.3 – 4.6 to evaluate if the beads withstand the extraction conditions.
Representative Results
EPS extraction
The appearance of granules after applying different EPS extraction procedures is shown in Figure 1. The shape and gel structure of granules were intact after centrifugation (Figure 1a) and EDTA extraction (Figure 1c). Granules were broken into fragments of different sizes by sonication. The turbidity in the liquid phase could be due to suspension of small fragments (Figure 1b) as the turbidity highly decreased after centrifugation. Formamide and formaldehyde alone did not have any impact on changing the shape of the granule and its gel structure (data not shown). After the addition of NaOH, the liquid phase turned yellowish. Some fluffy material was detached from the surface of the granules and formed a layer on top of the settled granules (Figure 1d and 1e). Still, the shape of the granules was not changed. The addition of NaOH apparently improved EPS solubilization, but could not damage the gel matrix structure. In comparison, granules completely disappeared after Na2CO3 extraction (Figure 1f). Instead a mixture of sol-like liquid and tiny jelly-like particles were formed, showing the gel matrix of granules was indeed solubilized.
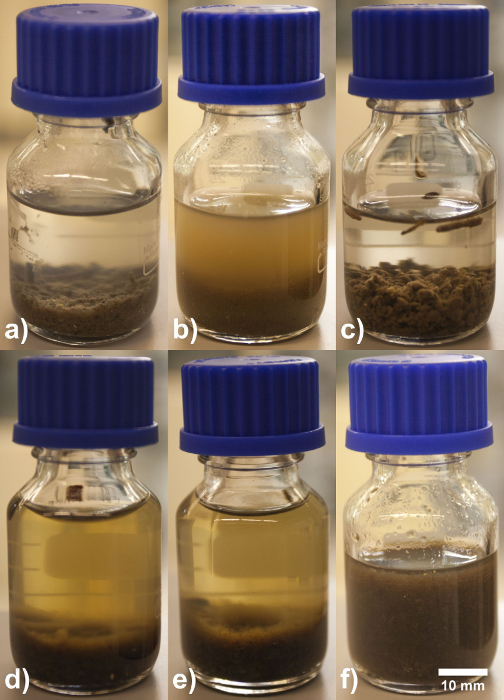
Figure 1. Aerobic granular sludge EPS extractions. For a better visualization of the impact of each extraction method on the granules, experiments were conducted in 25 ml glass bottles. After the extraction procedure, the extracts were kept for 1 hr at room temperature to allow suspended matter to settle. (a) Centrifugation extraction, (b) Sonication extraction, (c) EDTA extraction, (d) Formamide – NaOH extraction, (e) Formaldehyde – NaOH extraction, (f) High temperature – Na2CO3 extraction. Please click here to view a larger version of this figure.
EPS yield with respect to the VS fraction for each method is illustrated in Figure 2. The yield is presented in mg VSEPS per g initial VSgranule. The amount of EPS obtained by formaldehyde + NaOH, formamide + NaOH and Na2CO3 + heat + mixing was higher than that of centrifugation, sonication and EDTA extraction. Similar results for these extraction techniques were also shown by previous studies11–13,15 indicating that alkaline conditions enhance EPS solubility26,27. The amount of EPS recovered by Na2CO3 was the highest, more than 20 times of that obtained only by centrifugation. Additionally, the total EPS yield of the Na2CO3 extraction can be further enhanced by multiple extractions. A second extraction using the pellet discarded in step 1.6.8 (protocol section) of the first extraction increased the total yield by 28%, a quadruple extraction even increased the total yield by 46%.
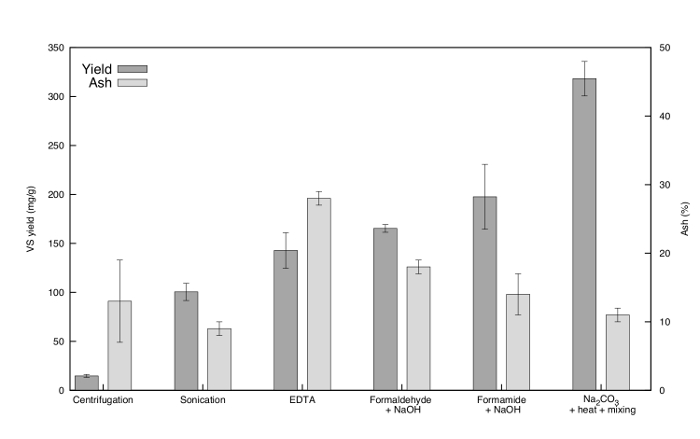
Figure 2. Results of all extraction methods with respect to VS yield and ash content. For each extraction the first bar represents the VS yield in mg VSEPS per g initial VSgranule. The second bar represents the weight percentage of ash in the extracted TS. The error bars illustrate the standard deviation of the three extractions performed for each extraction technique. Please click here to view a larger version of this figure.
Alginate-like extracellular polymer (ALE) extraction
After the pH of the EPS extracted by the Na2CO3 extraction was adjusted to 2.2, 63% of the total VS was precipitated. The precipitate is acidic ALE25. The residual fraction was likely EPS which can be solubilized under the extraction conditions, but cannot form a precipitate at pH 2.2.
Ionic hydrogel formation test
Aerobic granules have been described as being similar to a hydrogel. The granulation process has been regarded as a gel-forming phenomenon with glycosides as the gelling agent4,9,25,28. Normally, Ca2+ is one of the most common cations in wastewater. In addition, it easily binds with acidic polysaccharides (e.g., alginates and poly-galacturonic acid), presumably as a counter-ion to mediate gelation29. Thus resulting in an ionically cross-linked hydrogel. It was observed that the addition of Ca2+ ions can accelerate aerobic sludge granulation30. Therefore, Ca2+-EPS (ionic hydrogel) could play an important role in building up the gel matrix structure in aerobic granular sludge. In this respect, whether the extracted EPS forms an ionic hydrogel with Ca2+ ions could be used as a test to check if the extracted EPS is a structural polymer contributing to the formation of the gel matrix in aerobic granular sludge9.
In this research, for the EPS extracted from AGS (Figure 3a) by various methods, only the EPS extracted by Na2CO3 held the shape of a droplet in 2.5% (w/v) CaCl2 solution and formed stable ionic hydrogel beads. Moreover, the sodium ALE obtained from this EPS by additional steps (ALE polymer extraction, Figure 3b) displayed the same property as well. The color and morphology of the Ca2+-ALE gel beads (Figure 3c) are similar to aerobic granular sludge (Figure 3a). Apparently, the EPS extracted by the Na2CO3 method contributes to the formation of the gel matrix in aerobic granular sludge. ALE, which is a main component of this EPS are structural polymers, able to form an ionic hydrogel.
Stability test of the ionic hydrogel
It was observed that during EPS extraction, aerobic granules kept their spherical shape in EDTA, formaldehyde + NaOH and formamide + NaOH (Figure 1). In order to understand if the extracted structural polymers play a role in the stability of the granules, Ca2+-ALE beads were treated exactly the same way as aerobic granules during the extraction. Interestingly, Ca2+-ALE beads displayed the similar stabilities as that of AGS (Figure 3d – 3f), i.e., Ca2+-ALE beads were extremely stable in EDTA. There was little amount of ALE detached from the surface of Ca2+-ALE beads (tiny brownish floc in Figure 3e and 3f), when the Ca2+-ALE beads had been soaked in formaldehyde + NaOH and formamide + NaOH for three hours, respectively. This similarity in terms of stability between Ca2+-ALE beads and aerobic granules indicates that ALE are one part of the important structural polymers forming the AGS gel matrix.
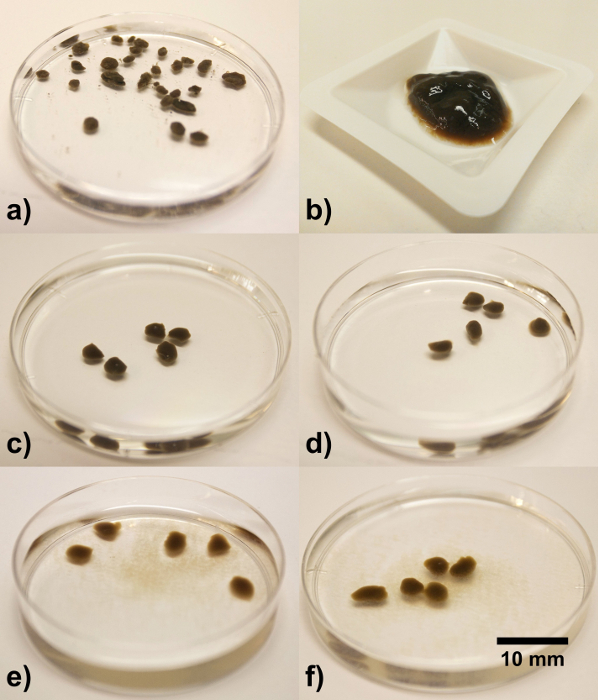
Figure 3. Aerobic granules and extracted ALE. (a) Granules in demineralized water prior extraction. (b) Acidic ALE (extracted according to the paragraphs 1.6 and 2) after centrifugation at 4,000 × g and 4 °C for 20 min. Results of the stability test of the ionic hydrogel. (c) Ca2+-ALE-beads stored in demineralized water for 4 hr at 4 °C. (d) Ca2+-ALE-beads stored in 2% EDTA, for 3 hr at 4 °C. (e) Ca2+-ALE-beads stored in formamide + NaOH for 4 hr at 4 °C. (f) Ca2+-ALE-beads stored in formaldehyde + NaOH for 4 hr at 4 °C. Please click here to view a larger version of this figure.
Discussion
Remarks for the protocol section
The extraction of EPS/ALE is described for a volume of 50 ml and 3 g of granules. These values are intended as guidelines. Extractions with higher granule concentrations can decrease the yield of the extracted EPS. During the extraction of ALE the temperature should be kept constant at 80 °C for 30 min. The time required for the mixture to heat up (around 5 min) is included in the protocol. Furthermore, the extraction efficacy is enhanced by using a magnetic stir bar of the same size as the diameter of the flask bottom. This will result in good mixing properties and milling effects, promoting the extraction of EPS.
Later in the protocol section, TS and VS yields of all extractions (supernatant collected in steps 1.1-1.6) are determined. Dialysis needs to be performed prior to TS and VS measurement to decrease possible errors owing to the presence of chemicals used for extractions. A MWCO of 3,500 Da is recommended to remove these chemicals while retaining the EPS macromolecules within the dialysis bag. The dialysis bag should have a larger volume than the volume of the extract. This is necessary, because the volume of the extract will increase during the dialysis (e.g., for EDTA extraction up to 40% volume increase). The extent of chemical removal by dialysis can be determined by measuring the pH in the sample prior and after dialysis. Alternatively, conductivity measurements of the dialysis water show the extent of ion removal.
To obtain ALE from the total extracted EPS (steps 1.6 and 2) the dialysis step is optional. Nevertheless, dialysis has three benefits: it reduces the amount of HCl needed for the precipitation, it improves the acid mass transfer in the extract and decreases the ash content of the obtained ALE. For the precipitation of ALE it is recommended to use a glass beaker with a much larger volume than the extract. Na2CO3 is normally overdosed in the extraction. The added HCl will first react with the Na2CO3 left in the extract, resulting in carbon dioxide formation and, if the sample was not dialyzed before, in foaming. During the addition of HCl, the extract should be stirred slowly with a magnetic stir bar of the same size as the bottom of the beaker. A stir bar of this size and slow stirring will result in even mixing without breaking the structure of the precipitate. If acidic gel clumps are formed in the extract, the beaker should be swirled slightly by hand. The precipitation is conducted with an acid concentration of 1 M to avoid a large volume increase of the extract while still obtaining a homogeneous distribution of the acid in the sample. Higher acid concentrations can result in a regional pH decrease and acidic gel clumps formation. A pH lower than 2.0 decreases the amount of ALE that can be recovered, probably due to structural changes of the polymers at lower pH. It is therefore important to keep the final pH at 2.20 ± 0.05.
Limitations
The ALE extraction method aims to extract structural extracellular polymers of the EPS from AGS or biofilms in general and is not intended to extract all present EPS. To extract all EPS, a combination of more than one extraction method is necessary. Moreover, as shown with the increase of the VSEPS yield by applying a double and quadruple extraction, one single extraction will not extract all structural EPS. ALE extraction is a harsh EPS extraction method, combining constant mixing with heat and alkaline conditions. For this reason it is possible that some intracellular material is extracted together with the EPS. Although cell lysis can be caused by physical and chemical extraction techniques (sonication31,32, NaOH31,32, EDTA11,32, CER32, heat32 and high shear rates by mixing19), the presence of intracellular material in recovered EPS still needs to be verified. The ionic gel-forming property of the extracted EPS is the main focus of this research, whether the recovered EPS contains intracellular material was not analyzed. Future research will focus on identifying intracellular material in the extracted EPS.
Solubilizing the hydrogel matrix of AGS is crucial to extract structural EPS
EPS forms a dense and compact hydrogel matrix in AGS. Although EPS contains various classes of organic macromolecules such as polysaccharides, proteins, nucleic acids, (phospho)lipids, humic substances and some intercellular polymers7,5,8, not all of them form a gel. Only those gel-forming polymers are here considered as structural polymers in EPS.
The aim of EPS extractions is to first solubilize EPS and then to collect the solubilized EPS. If the structural EPS (i.e., the EPS forming a hydrogel) is the target of the extraction, the gel matrix of AGS has to be solubilized first. Only methods that can solubilize the gel matrix are capable of extracting structural EPS. In this research, some frequently used EPS extraction methods such as centrifugation10–15, sonication10,14,15, EDTA10–12,14,15, formaldehyde + NaOH10–15 and formamide + NaOH13 could not efficiently isolate the structural EPS. This is due to the fact that the hydrogel matrix of the aerobic granules was not solubilized by these methods. For this reason, stability tests in section 4 were only performed with conditions present in EDTA, formamide + NaOH and formaldehyde + NaOH extraction. These three extractions were not capable of isolating structural EPS, but still obtained the highest VSEPS yield besides the Na2CO3 extraction. Conditions of the Na2CO3 extraction were not applied as this extraction method clearly solubilized the AGS matrix. Hence the applied conditions during the stability test were considered representative.
Extraction with cation exchange resin (CER), another frequently used EPS extraction method, was not considered for this comparison, as previous studies on EPS extraction with CER did not yield better results than the chemical extractions used here.
Gel-forming EPS in AGS
Gel-forming EPS are considered as the structural EPS in the hydrogel matrix of AGS. It is worth pointing out that there are various kinds of hydrogels such as ionic gels, temperature-induced gels and pH induced gels. This study only focuses on EPS that form ionic gels. Regarding the large fraction of structural gel material extracted, this is likely to be the dominant structural EPS. There are certainly possibilities that other kinds of EPS that form different kinds of hydrogels (e.g., pH induced gel28) exist in the same or other type of aerobic granules. Nevertheless, no matter what kind of hydrogel is targeted, solubilizing the EPS gel matrix is the most important step to extract gel-forming EPS.
Currently, little research has been done on structural EPS of granular sludge. The ALE extraction described in this protocol is capable of extracting gel-forming EPS from AGS and will be used in future studies to characterize structural EPS. More research needs to be done on AGS, structural EPS and non-structural EPS to better understand the process and function of granulation and EPS. Especially the following three points need to be investigated: why microorganisms produce such a large amount of EPS, what is the exact composition of EPS and how is the composition of EPS modified depending on environmental changes. Detecting and analyzing all involved compounds and their interactions will help to understand biofilms and how to use them to our advantage.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was financially supported by the SIAM Gravitation Grant 024.002.002, the Netherlands Organization for Scientific Research and by the Dutch Technology Foundation (STW – Simon Stevin Meester 2013). The authors want to thank Mario Pronk for providing the granular sludge samples.
Materials
| 250 ml baffled flask | Kimble | 25630-250 | |
| 1000 ml glass beaker | VWR | 213-1128 | |
| RCT basic, magnetic stirrer with thermometer | IKA | 3810000 | |
| sodium carbonate decahydrate | Merck KGaA | 1063911000 | |
| 50 ml centrifugation tubes | greiner bio-one | 227261 | |
| Multifuge 1 S-R, centrifuge | Heraeus/Thermo Scientific | – | |
| hydrochloric acid, 37 % | Sigma-Aldrich | 30721-1L-GL-D | |
| 250 ml glass beaker | VWR | 213-1124 | |
| calcium chloride dihydrate | Merck KGaA | 1023821000 | |
| 1 ml Pasteur Pipette | Copan | 201C |
References
- Pronk, M., de Kreuk, M. K., de Bruin, B., Kamminga, P., Kleerebezem, R., van Loosdrecht, M. C. M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 84, 207-217 (2015).
- Kreuk, M. K., Kishida, N., van Loosdrecht, M. C. M. Aerobic granular sludge – state of the art. Water Sci. Technol. 55 (8-9), 75 (2007).
- Adav, S. S., Lee, D. J., Show, K. Y., Tay, J. H. Aerobic granular sludge: Recent advances. Biotechnol. Adv. 26, 411-423 (2008).
- Seviour, T., Pijuan, M., Nicholson, T., Keller, J., Yuan, Z. Understanding the properties of aerobic sludge granules as hydrogels. Biotechnol. Bioeng. 102 (5), 1483-1493 (2009).
- Flemming, H. -. C., Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8 (9), 623-633 (2010).
- Seviour, T., Yuan, Z., van Loosdrecht, M. C. M., Lin, Y. Aerobic sludge granulation: A tale of two polysaccharides?. Water Res. 46 (15), 4803-4813 (2012).
- Wingender, J., Neu, T. R., Flemming, H. -. C. What are Bacterial Extracellular Polymeric Substances. Microb. Extracell. Polym. Subst. Charact. Struct. Funct. , 27-53 (1999).
- Flemming, H. -. C., Neu, T. R., Wozniak, D. J. The EPS Matrix: The "House of Biofilm Cells.". J. Bacteriol. 189 (22), 7945-7947 (2007).
- Lin, Y. M., Sharma, P. K., van Loosdrecht, M. C. M. The chemical and mechanical differences between alginate-like exopolysaccharides isolated from aerobic flocculent sludge and aerobic granular sludge. Water Res. 47 (1), 57-65 (2013).
- Fang, H. H. P., Jia, X. S. Extraction of extracellular polymer from anaerobic sludges. Biotechnol. Tech. 10 (11), 803-808 (1996).
- Liu, H., Fang, H. H. P. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 95, 249-256 (2002).
- Comte, S., Guibaud, G., Baudu, M. Effect of extraction method on EPS from activated sludge: An HPSEC investigation. J. Hazard. Mater. 140 (1-2), 129-137 (2007).
- Adav, S. S., Lee, D. J. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure. J. Hazard. Mater. 154, 1120-1126 (2008).
- Pan, X., Liu, J., Zhang, D., Chen, X. I., Li, L., Song, W., Yang, J. A comparison of five extraction methods for extracellular polymeric substances (EPS) from biofilm by using three-dimensional excitation-emission matrix (3DEEM) fluorescence spectroscopy. Water SA. 36 (1), 111-116 (2010).
- D’Abzac, P., Bordas, F., Van Hullebusch, E., Lens, P. N. L., Guibaud, G. Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: Comparison of chemical and physical extraction protocols. Appl. Microbiol. Biotechnol. 85 (5), 1589-1599 (2010).
- Comte, S., Guibaud, G., Baudu, M. Relations between extraction protocols for activated sludge extracellular polymeric substances (EPS) and EPS complexation properties: Part I. Comparison of the efficiency of eight EPS extraction methods. Enzyme Microb. Technol. 38 (1-2), 237-245 (2006).
- Adav, S. S., Lee, D. J., Tay, J. H. Extracellular polymeric substances and structural stability of aerobic granule. Water Res. 42, 1644-1650 (2008).
- Caudan, C., Filali, A., Lefebvre, D., Spérandio, M., Girbal-Neuhauser, E. Extracellular polymeric substances (EPS) from aerobic granular sludges: Extraction, fractionation, and anionic properties. Appl. Biochem. Biotechnol. 166 (7), 1685-1702 (2012).
- Frølund, B., Palmgren, R., Keiding, K., Nielsen, P. H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 30 (8), 1749-1758 (1996).
- Nielsen, P. H., Jahn, A. Extraction of EPS. Microb. Extracell. Polym. Subst. Charact. Struct. Funct. , 49-72 (1999).
- Lin, Y. M., Nierop, K. G. J., Girbal-Neuhauser, E., Adriaanse, M., van Loosdrecht, M. C. M. Sustainable polysaccharide-based biomaterial recovered from waste aerobic granular sludge as a surface coating material. Sustain. Mater. Technol. 4, 24-29 (2015).
- Lin, Y. M., Wang, L., Chi, Z. M., Liu, X. Y. Bacterial Alginate Role in Aerobic Granular Bio-particles Formation and Settleability Improvement. Sep. Sci. Technol. 43 (7), 1642-1652 (2008).
- . . Standard Methods for the Examination of Water and Wastewater. , (1998).
- Mchugh, D. J. . A guide to the seaweed industry. , (2003).
- Lin, Y., de Kreuk, M., van Loosdrecht, M. C. M., Adin, A. Characterization of alginate-like exopolysaccharides isolated from aerobic granular sludge in pilot-plant. Water Res. 44 (11), 3355-3364 (2010).
- Zorel, J. A., Aquino, S. F., Sanson, a. L., Castro-Borges, W., Silva, S. Q. Evaluation of EPS extraction protocols from anaerobic sludge for gel-based proteomic studies. Water Sci. Technol. 72 (4), 535 (2015).
- Ruiz-Hernando, M., Cabanillas, E., Labanda, J., Llorens, J. Ultrasound, thermal and alkali treatments affect extracellular polymeric substances (EPSs) and improve waste activated sludge dewatering. Process Biochem. 50 (3), 438-446 (2015).
- Seviour, T., Pijuan, M., Nicholson, T., Keller, J., Yuan, Z. Gel-forming exopolysaccharides explain basic differences between structures of aerobic sludge granules and floccular sludges. Water Res. 43, 4469-4478 (2009).
- de Kerchove, A. J., Elimelech, M. Formation of polysaccharide gel layers in the presenceof Ca2+ and K+ ions: Measurements and mechanisms. Biomacromolecules. 8 (1), 113-121 (2007).
- Jiang, H. L., Tay, J. H., Liu, Y., Tay, S. T. L. Ca2+ augmentation for enhancement of aerobically grown microbial granules in sludge blanket reactors. Biotechnol. Lett. 25 (2), 95-99 (2003).
- Liang, Z., Li, W., Yang, S., Du, P. Extraction and structural characteristics of extracellular polymeric substances (EPS), pellets in autotrophic nitrifying biofilm and activated sludge. Chemosphere. 81 (5), 626-632 (2010).
- Guo, X., Liu, J., Xiao, B. Evaluation of the damage of cell wall and cell membrane for various extracellular polymeric substance extractions of activated sludge. J. Biotechnol. 188, 130-135 (2014).

