Generation of Murine Monoclonal Antibodies by Hybridoma Technology
Summary
An optimized protocol is presented for the generation of monoclonal antibodies based on the hybridoma technology. Mice were immunized with an immunoconjugate. Spleen cells were fused by PEG and an electric impulse with immortal myeloma cells. Antibody-producing hybridoma cells were selected by HAT and antigen-specific ELISA screening.
Abstract
Monoclonal antibodies are universal binding molecules and are widely used in biomedicine and research. Nevertheless, the generation of these binding molecules is time-consuming and laborious due to the complicated handling and lack of alternatives. The aim of this protocol is to provide one standard method for the generation of monoclonal antibodies using hybridoma technology. This technology combines two steps. Step 1 is an appropriate immunization of the animal and step 2 is the fusion of B lymphocytes with immortal myeloma cells in order to generate hybrids possessing both parental functions, such as the production of antibody molecules and immortality. The generated hybridoma cells were then recloned and diluted to obtain stable monoclonal cell cultures secreting the desired monoclonal antibody in the culture supernatant. The supernatants were tested in enzyme-linked immunosorbent assays (ELISA) for antigen specificity. After the selection of appropriate cell clones, the cells were transferred to mass cultivation in order to produce the desired antibody molecule in large amounts. The purification of the antibodies is routinely performed by affinity chromatography. After purification, the antibody molecule can be characterized and validated for the final test application. The whole process takes 8 to 12 months of development, and there is a high risk that the antibody will not work in the desired test system.
Introduction
The hybridoma technology presented in this protocol was first described by Köhler and Milstein1 in 1975 and, except for some technical improvements, the main procedure has not changed dramatically during the last 40 years2. The aim of this protocol is to explain a more appropriate immunization strategy, a standard method for the generation of monoclonal antibodies, and an example for a validation method (ELISA).
Antibodies are incredible tools and contribute to a wide range of technological approaches, such as flow cytometry, magnetic cell sorting, or immunofluorescence, as well as to diagnostic and therapeutic options for disease monitoring and treatment3. The commercial availability of monoclonal antibodies for desired targets is demonstrated by the presence of over 24 different web databases with nearly countless quantities of antibodies or antibody-related products4. In 2015, antibody molecules were part of an international discussion5-7 due to serious problems in proper validation and characterization of commercially available antibodies.
It can be difficult and expensive to find specific antibodies for a targeted antigen, and often, they do not have the affinity or specificity needed. Although generating an antibody is still time-consuming and requires skilled personnel to develop and validate the antibody, producing an antibody individually might better than buying one.
Due to the fact that antibody production is time-consuming and requires experience, alternative methods for the production of binding molecules were developed to overcome these problems. The most commonly used alternative method is the recombinant production of single-chain antibodies via phage display. The genes for the variable binding region are extracted from cells and combined with the coating protein of a phage. The single chain is then expressed on the surface of a bacteriophage and screened in several panning steps8. The production of single-chain antibodies is a bit faster, but it also requires a skilled experimentalist. The disadvantages of some recombinant single-chain antibodies are poor stability and a lack of suitability for in vitro diagnostics. In most diagnostic tests, an Fc-receptor for detection is necessary, which needs to be added to a recombinant single-chain antibody afterwards. Again, this is time-consuming and even more complex than the hybridoma technique. In in vitro diagnostics, full-length monoclonal mouse and rabbit antibodies have been demonstrated to be the best choice.
One of the major steps in generating monoclonal antibodies must be done before the work in the lab starts: the design of the immunoconjugate. Questions that need to be addressed are: What is the physical composition of the target in the final application, and which matrices are present? Which concentration will the target have in the application? What is the final application, and what are the requirements that the antibody must fulfill?
Always take into account that if a linear peptide fragment is used, it also has to be linear in the final epitope in the target of choice; otherwise, the antibody will not bind. Of course, independent of the screening method, antibodies could be selected to recognize different antigenic formats in different applications, but this must be validated very precisely. These are the reasons why antibody development and validation are such ambitious processes.
The choice of the antigenic format for immunization is fundamental for antibody development and determines the success or failure of this process. Once the mice express a relevant antibody titer, the spleen cells are isolated and fused with myeloma cells. The most common myeloma cell lines for murine monoclonal antibody development are X63-Ag 8.6539 and Sp2/0-Ag 1410 from a Balb/c mouse strain. The cells descend from a malignant B cell lymphoma and were selected because they do not secrete any of their own heavy or light chains. The cells can be adapted to a ratio between 1:10 and 10:1 (splenocytes versus myeloma cells). In this protocol, the cells were adapted to a ratio of 3:1 and fused by polyethylene glycol (PEG) and electrofusion, according to Stoicheva and Hui11.
The fusion of B cells and myeloma cells is a random process. Therefore, hybrids of two B lymphocytes or two myeloma cells could be generated, but those hybrids would not be able to survive for a long time in culture. The cells undergo a hypoxanthine, aminopterin, and thymidine (HAT) selection, by which only fused hybridoma cells can survive due to the possibility of using the de novo pathway of pyrimidine synthesis. For the generation of monoclonal antibodies, it is necessary to obtain a cell line originating from one mother cell. The monoclonality is ensured by limiting the dilution techniques and the microscopic analysis of cell growth. The hybridoma culture supernatants are screened for specific antibody production, mostly by ELISA or flow cytometry, and the best binders are selected. After mass culture and purification, the antibody molecule can finally be characterized and validated for the desired application.
Protocol
Balb/c or NMRI mice (Mus musculus) from our breeding colony at the University of Potsdam (Potsdam, Germany) were used for the production of monoclonal antibodies. The animal work was conducted according to relevant national and international guidelines. The study was approved by the Brandenburg Ministry of Environment, Health, and Consumer Protection (reference number V3-2347-A16-4-2012).
1. Preparation of Immunoconjugates
- For coupling the antigen to a carrier, use standard coupling protocols via amino-, carboxy, or sulfo-groups, such as by glutaraldehyde, N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC), or N-[y-maleimidobutyryloxy]succinimide ester (sulfo-GMBS).
- For coupling (e.g., with glutaraldehyde)13, use the target protein or peptide and the carrier (ovalbumin, bovine serum albumin, or keyhole limpet hemocyanin) in a 1:1 ratio. Dilute the target protein or peptide in phosphate-buffered saline (PBS) and the carrier protein in 50 mM NaHCO3 buffer. Adapt the volume and the concentration to the amount necessary for immunizations.
- Mix both solutions and add 1.25% glutaraldehyde. Mix and incubate the sample for 4 h at room temperature (RT). To select the hybridoma cells, make the same immunoconjugate, but with another carrier, in order to avoid the selection of unspecific carrier binders.
- Dialyze the coupling sample by performing a fast dialysis with commercial coarse resin (e.g., Sephadex G25).
- Perforate the bottom of a 1.5-mL reaction vial with a hollow needle and place it in a 15-mL tube. Put glass wool in the bottom for sealing and cover it with 300 mg of coarse resin (to the calibration mark of 0.5 mL).
- Carefully, put 1 mL PBS dropwise into a 1.5-mL reaction tube and make sure that the glass wool is still sealing the perforation. Let the coarse resin swell and centrifuge the column at 500 x g for 2 min. One such column can be used to dialyze 200 µL of the coupling sample. For higher volumes, make more columns.
- Change the tube and drop the coupling solution onto the swelled coarse resin. Centrifuge again at 500 x g for 2 min. Remove the eluent from the tube and use it for immunization.
2. Immunization of Animals
- Choose two or three 6- to 12-week-old Balb/c mice for immunization.
- For one animal, prepare 150 – 200 µg of the immunoconjugate in 200 µL of PBS and mix it with 100 µL of complete Freund´s adjuvant (CFA). Mix the solution 3 times with a short and thin needle (0.6 mm diameter) on a 1-mL syringe. Inject the solution intraperitoneally into the mouse.
- Give a booster injection 6 weeks later with the same amount of the immunoconjugate, but without CFA. Take blood 7 days later (out of the retroorbital sinus) and prepare the serum by incubating the blood for 3 – 4 h at room temperature (RT).
- Centrifuge the mixture at 3,000 x g for 5 min. Take the serum supernatant and transfer it into a new tube. Store it at -20 °C. Discard the pellet.
- Analyze the sera via ELISA, as described in Step 3. For fusion, choose the mouse with the highest serum titer.
- In preparation for cell fusion, boost the mouse with 150 – 200 µg of the immunoconjugate in 300 µL of PBS without adjuvant only 4 days before the fusion.
3. ELISA
- Prepare an antigen solution with a concentration of 5 µg/mL in PBS and transfer 50 µL into each ELISA well of a 96-well plate.
- Incubate the plate for 2 h at 37 °C or overnight at 4 °C in a humid chamber.
- Wash the plate 3 times with tap water. Block the wells with 80 µL of PBS/5% neonatal calf serum (NCS) per well for 1 h at RT in a humid chamber. Prepare the serum dilutions in a 1:5 series starting with 1:50. For analysis of the cell culture, use the undiluted culture supernatant.
- Wash the plate 3 times with tap water. Pipette 50 µL of the sample per well and incubate for 1 h at RT in a humid chamber. Prepare the secondary antibody solution in a 1:5,000 dilution of a goat anti-mouse IgG antibody conjugated to horseradish peroxidase (HRP).
- Wash the plate 3 times with tap water and add 50 µL of the secondary antibody solution per well. Incubate for 45 min at RT in a humid chamber.
- Prepare a fresh substrate solution. Make stock solutions of 0.1 M NaH2PO4 and 0.1% hydrogen peroxide. Take 5 parts NaH2PO4, 4 parts hydrogen peroxide, and 1 part tetramethylbenzidine for the fresh substrate solution.
- Wash the plate 10 times with tap water. Pipette 50 µL of fresh substrate solution into the wells and incubate for 5 – 10 min in the dark.
- Stop the reaction with 50 µL 1 M H2SO4 per well. Using a plate reader, measure the optical density at 450 nm with a reference wavelength at 630 nm.
4. Preparation of Myeloma Cell Cultures
- From now on, perform all work under sterile conditions.
- Prepare a fresh culture medium for cell cultivation. Supplement 500 mL of RPMI1640 with 10% fetal calf serum (50 mL), 2 mM L-glutamine (5 mL), and 50 µM mercaptoethanol (5 mL). Substitute the glutamine every 7 days.
- Thaw a vial of murine SP2/0 cells in warm water. Transfer the cell solution into 10 mL of fresh culture medium and centrifuge for 10 min at 200 x g. Discard the supernatant and resuspend the pellet in 1 mL of fresh culture medium.
- Transfer the cells in a 25-cm2 flask and incubate them at 37 °C and 5% CO2 in an incubator. Expand the cells to two 75-m2 flasks before fusion. Conserve aliquots for long-term storage, as described in Step 7.
5. Preparation of Feeder Cells
- Sacrifice a 12-week-old NMRI mouse (according to national and institutional guidelines; e.g., by CO2 inhalation). Remove the fur and clean the abdomen with 70% ethanol.
- Inject 5 mL of ice cold RPMI medium into the abdomen, massage the abdomen with a tweezer, and aspirate the feeder cell suspension. Place the cells in an empty 50-mL tube. Perform this step once again. 7 to 10 mL of the feeder cell suspension should be retrieved.
- Prepare a 100-mL feeder cell suspension by adding 90 mL of full cell culture medium (RPMI 1640, 10% fetal calf serum, 2 mM glutamine, and 50 µM mercaptoethanol). Mix it and transfer 100 µL/well into 10 x 96-well cell culture plates. Incubate the plates overnight at 37 °C and 5% CO2 in an incubator.
6. Cell Fusion
- Sacrifice the immunized mouse by cervical dislocation or CO2 inhalation and excise the spleen14. Take additional blood from the heart and prepare the serum, as described in step 2.3, as a positive control in the ELISA.
- Prepare a spleen cell suspension by pressing the spleen through a cell strainer. Fill up to 20 mL with full cell culture medium. Harvest the cells in 50-mL tubes by centrifugation (200 x g, 10 min, and 4 °C). Discard the supernatant.
- Rinse the cell culture flask and harvest the myeloma cells by centrifugation (200 x g, 10 min, and 4 °C) and discard the supernatant.
- Resuspend the cell pellets in 20 mL of balanced salt buffer (BSS) (125 mM NaCl, 5 mM KCl, 4 mM CaCl2, 2.5 mM MgCl2, and 5 mM Tris-HCl; pH 7.4) and centrifuge again. Repeat the washing steps three times.
- For adjusting the splenocyte:myeloma cell ratio, count the cells after the second washing step by first resuspending the pellet in 1 mL of BSS buffer. Make a 1:10 dilution and transfer 10 µL to a cell counting chamber. Count the cells and determine the cell concentration in 1 mL.
- Adjust the cell numbers in the BSS buffer to three-parts splenocytes and one-part myeloma cells in a 1-mL final volume. Take 200 µL of the cell suspension and mix it with 200 µL of PEG8000 (1 g in 4 mL of BSS buffer). Transfer the 400 µL into an electroporation cuvette with a diameter of 2 mm and set the pulse (600 – 650 V and 25 ms).
- After the pulse, incubate the cells at RT for 3 min in the cuvette. Remove the cells and put them dropwise into an Erlenmeyer flask with 100 mL of HAT Medium (RPMI 1640, 10% FCS, 2 mM glutamine, 50 µM mercaptoethanol, 100 µM hypoxanthine, 5.8 µM azaserine, and 16 µM thymidine).
- Repeat the fusion 4 times until the 1-mL batch is spent. Incubate the Erlenmeyer flask for 3 h at 37 °C and 5% CO2 in an incubator.
- Supplement the cell suspension again with hypoxanthine, azaserine, and thymidine before plating the cells on the feeder layer. Add 100 µL of the cell suspension per well to the feeder layer plates and incubate the plates at 37 °C and 5% CO2 (in an incubator). The final concentration within the well should be 100 µM hypoxanthine, 5.8 µM azaserine, and 16 µM thymidine.
- Perform a microscopic analysis (magnification: 10X objective lens, 10X eye-piece) for clonal growth in each well of the plate after 7 days in order to determine if the wells are mono- or polyclonal. Monoclonal cells are one cluster of cells that originate from one single cell. Polyclonal cells are multiple clusters of cells in one well. Change the complete media after 7 days and incubate again for 7 days in HAT medium.
- Change the HAT medium and cultivate the cells in full cell culture medium. Use the supernatant to perform an ELISA, as described in Step 3. Positive hybridoma cells must be expanded in 24-well plates according to the tests for the production of specific antibodies. If the cells are polyclonal, a limited dilution has to be performed (described in step 8).
7. Cryoconservation of Hybridomas
- Cryoconserve positive mono- and polyclonal hybridomas for long-term storage. Harvest the cells from a 24-well plate with a confluence of > 60% by centrifugation (200 x g, 10 min, and 4 °C).
- Resuspend the pellet in 500 µL of full cell culture media and transfer it into a cryotube. Add 500 µL of freeze medium (RPMI 1640, 20% FCS, and 15% dimethylsulfoxide), mix it, and put it in in a Styrofoam box or a special freezing box. Store at -80 °C for 3 days. After 3 days, the cryotube can be transferred to liquid nitrogen for long-term storage.
8. Limiting Dilution of Polyclonal Hybridomas
- Make a feeder layer culture, as described in Step 5. Harvest positive polyclonal cells, as described in Step 7.1. Count the cells, as described in Step 6.5, and adjust the cell number to 10 cells/mL, with a final volume of 10 mL for a 96-well plate. Add 100 µL/well onto the feeder layer.
- Incubate for 7 days at 37 °C and analyze the clonal growth microscopically (magnification: 10X objective lens, 10X eye-piece). Make an ELISA of the culture supernatant, as described in Step 3, and identify the appropriate monoclonal hybridomas for mass culture.
9. Mass Culture and Purification of Monoclonal Antibodies
- Transfer the monoclonal hybridomas with the appropriate ELISA signals to 25 cm2 or 75 cm2 flasks and collect up to 500 mL of the culture supernatant. Test the culture weekly for specific antibody production and conserve 3 – 5 aliquots per hybridoma for long-term storage, as described in step 7.
- Centrifuge the culture supernatant at 4,900 x g and 4 °C for 15 min and filter it through a 0.45-µm filter. Mix the supernatant with binding buffer (4 M NaCl and 2 M glycine NaOH, pH 8.9) in a 3:1 ratio.
- Prepare a protein A column by washing it with washing buffer (dilute the binding buffer 1:3 in dH2O). Pass the supernatant from Step 9.2 over the column and collect the flow-through. Elute the bound antibody with 0.1 M citrate, pH 3.5 and neutralize the pH immediately with 500 µL of Tris-HCl, pH 9.0.
- Characterize the eluted antibody by SDS-PAGE, Western Blot, and ELISA, and validate it for the final application.
- For the SDS PAGE, take 3 – 5 µg of purified antibody solution per lane (a 20-µL sample), add 5 µL of 4x SDS sample loading buffer (8% SDS, 20% 2-mercaptoethanol, 40% glycerin, and 0.015% bromophenol, reduced), and heat the probes at 95 °C for 5 min. As a control, use the same sample with a purified antibody of the same isotype but irrelevant specificity.
- Load the samples on a 12.5% SDS gel, place 5 µL of an unstained protein ladder in an additional lane, and separate them by electrophoresis. Stain the gel with Coomassie Brilliant Blue and document the results. A basic SDS protocol was established by Laemmli in 197015.
Representative Results
Figure 1 shows an example of the antigen-specific serum titer for one immunization performed in the lab. In this figure, the immune sera A and B were titrated from 1:50 to 1:5,000,000 in comparison to a serum of a naïve mouse. The antigen was coated on the solid phase, and the specific antibodies were detected with a POD-conjugated goat anti-mouse Ig antibody. Both sera of the immunized mice showed a significantly higher antigen-specific titer compared to the naïve mouse. The signal at a dilution of 1:5,000 is sufficient to move on to the next step, the cell fusion.
The growth of hybridomas was visible after 7 to 10 days of HAT selection by the development of cell clones, as shown in Figure 2. The image was taken from a 96-well plate 10 days after fusion. The cell clones and the aminopterinemono- or polyclonality of the wells were monitored by individual microscopic analyses (magnification: 10X objective lens, 10X eye-piece). Monoclonal wells were labeled with a point on the top of the plate. When the plates were tested in ELISA, antigen-specific signals from monoclonal hybridomas could be easily identified. Positive hybridoma cultures were recloned, expanded, and cryoconserved until the culture was monoclonal, stable, and antigen-specific. The culture supernatant was collected during the time of cultivation in order to purify the produced monoclonal antibody via protein A-mediated affinity chromatography. Figure 3 shows a standard elution profile of a monoclonal antibody with an IgG1 isotype.
Purified monoclonal antibodies were further characterized by an SDS PAGE, as shown in Figure 4. At 50 and 25 kDa, the heavy and light chains were clearly visible, indicating the purification of an antibody molecule. Lane 2 showed a control antibody with the same isotype.
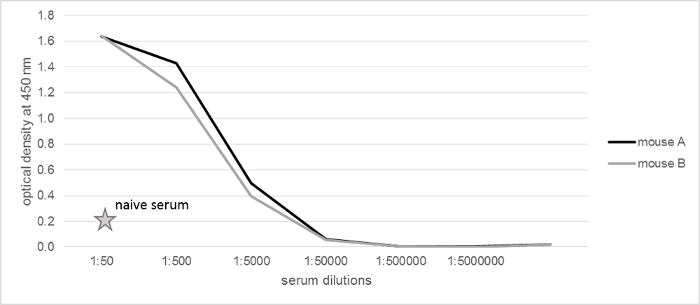
Figure 1: Serum Titration after Immunization. The sera from two mice were titrated in dilutions from 1:50 till 1:5,000,000 and tested for antigen specificity by an ELISA. As control serum of a non-immunized mouse was used in a dilution of 1:50. The antigen-specific signals were positive for both immunized mice and the splenocytes of mouse A were used for hybridoma generation. Please click here to view a larger version of this figure.
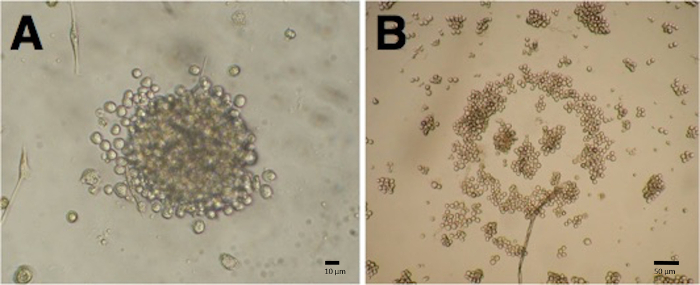
Figure 2: Photographs of Mono- and Polyclonal Hybridomas. Mono- and polyclonal hybridomas were microscopically analyzed 10 days after cell fusion. The pictures show the conventional outcome for stable hybridoma cell lines with monoclonal (A) and polyclonal (B) properties. A scale bar: 10 µm; B scale bar: 10 µm. Please click here to view a larger version of this figure.
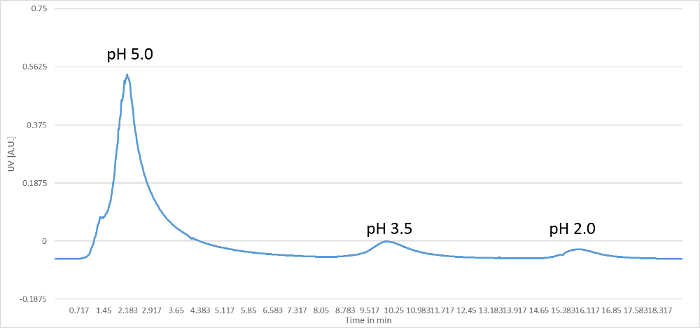
Figure 3: Standard Elution Profile for a Monoclonal Antibody with an IgG1 Isotype. The generated monoclonal antibody was purified via protein A-mediated affinity chromatography, and the elution profile of an IgG1 antibody is shown in the figure. The first peak indicates the eluted monoclonal antibody molecule at pH 5.0. The following peaks indicate pH 3.5 and pH 2.0. The pH reduction is necessary to completely clean the column of all substances and to regenerate the column matrix for the next purifications. Please click here to view a larger version of this figure.
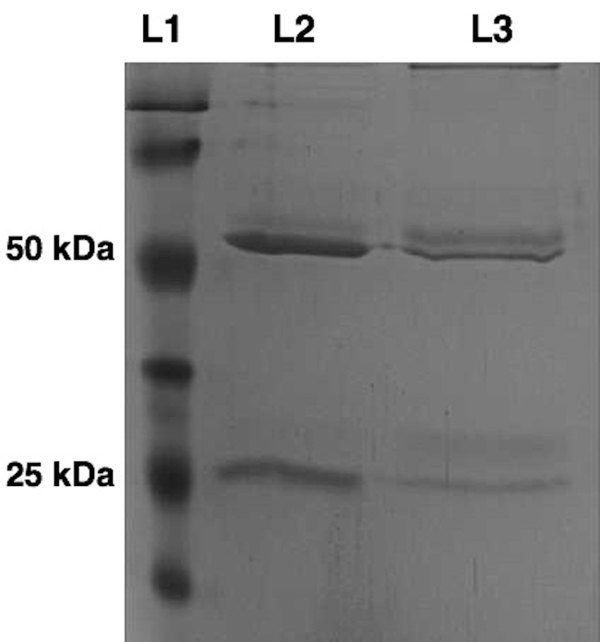
Figure 4: Standard SDS PAGE for a Monoclonal Antibody with an IgG1 Isotype. The purity of the antibody eluent was determined by a Coomassie-stained SDS-PAGE. 5 µg of the antibody solution were applied to L2 and compared to 5 µg of a standard IgG1 antibody in L3 under reduced conditions. Please click here to view a larger version of this figure.
Discussion
The generation of monoclonal antibodies by hybridoma technology requires an intense and detailed epitope analysis, especially with regard to the final application, when the antibody should recognize the target. This is often underestimated by users and leads to antibodies with weak performances. The fusion process is always random, which means that the outcome of specific hybridomas is highly dependent upon the cell ratio and the vitality at this point. After limited dilution, the cells are very unstable and require stringent monitoring of cell growth and antibody production. Those parameters should be carefully analyzed when troubleshooting the method.
Further limitations of the technique include the time needed for cell line generation and the selection of the desired antibody-producing cell. Due to the tetraploid set of chromosomes after fusion, the cells are very unstable and afterwards reduce the set to haploid again. This could lead to the loss of antibody-related genes, causing the cells to not produce antibodies anymore. Another limitation is that the selection of the desired antibody-producing cells is based on the culture supernatant and not on the purified antibody solutions. Therefore, a final characterization of the antibody is, in most cases, only possible after mass culture of the selected hybridoma clone. Thus, the risk that the antibody is not able to work in the final application is very high.
Several technological modifications, such as laser-enabled analysis and processing technology (LEAP)16, affinity capture surface display (ACSD)17, or gel microdrop technology18, were described in order to circumvent the drawbacks during the selection of suitable antibodies. All these methods are able to improve different aspects of hybridoma generation and selection, but most of them require costly equipment or optimization for every single cell line16.
Another improvement or modification that was developed in our lab is a novel immunization strategy with chimeric viral-like particles (VLPs), as described in Messerschmidt et al19. The particles can be modified by the desired epitope sequences embedded in the viral coat structure and presented on the surface of those particles. This leads to very fast and specific immunization after 4 weeks in the corresponding animals. The generated monoclonal antibodies have IgG2 or IgG1 isotypes and are dependent upon the epitope selection highly specific for the native antigen. We also developed a specific selection step for hybridoma cells by using artificial cell surface markers. The used transgenic myeloma cells display a stably inserted surface marker. This surface marker can be used to antigen- or isotype-specifically sort hybridoma cells directly after fusion, without limiting the dilution and without excessive ELISA screening, as is actually necessary in conventional hybridoma technology20.
As mentioned above, there are some promising alternatives available in monoclonal antibody generation that, in the future, could improve the handling and reduce the drawbacks of the current hybridoma technology. Nevertheless, the procedure will always require the detailed identification of the desired epitope in combination with a stringent and carefully planned application so that the generated monoclonal antibody works successfully. The generation of monoclonal antibodies by hybridoma technology will be an important and necessary step in the future due to the enormous demand in science, diagnostics, and therapy.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the German Federal Ministry of Education and Research (BMBF, Grant No: 03IPT7030X, 03IPT703A, and 03IP703) for funding our projects, “Artificial immune reactions,” “Camelid antibodies,” and “Antibody technologies.” We thank Prof. Burkhard Micheel for proofreading the manuscript and for the helpful comments.
Materials
| glutaraldehyde | Sigma Aldrich | G5882 | |
| N-(3-dimethylaminopropyl)-N-ethylcarbodiimide (EDC) | Sigma Aldrich | 39391-10ML | |
| Sulfo-GMBS | Perbio Science Germany | 22324 | |
| ovalbumin | Sigma Aldrich | A5503 | |
| bovine serum albumin | Sigma Aldrich | A2153 | |
| keyhole limpet hemocyanin | Sigma Aldrich | H8283 | |
| Falcon tubes 15 mL | Biochrom GmbH | P91015 | |
| reaction vials, 1.5 mL | Carl Roth GmbH & C0.KG | CNT2.1 | |
| hollow needle | Carl Roth GmbH & C0.KG | C724.1 | |
| glass wool | Carl Roth GmbH & C0.KG | 6574.1 | |
| Sephadex G25 coarse | Sigma Aldrich | GE-17-0034-02 | |
| Freund´s adjuvant, complete | Sigma Aldrich | F5881-10ML | |
| ELISA plates, 96 well | Greiner bio-one | 655101 | |
| neonatal calf serum | Biochrom GmbH | S1025 | |
| TipOne Tips 1000 µL | Starlab | S1111-2021 | |
| Pipette tips 200 µL | Greiner bio-one | 739291 | |
| HRP-conjugated goat-anti-mouse IgG antibody | Dianova | 115-035-003 | |
| tetramethylbenzidine | Carl Roth GmbH & C0.KG | 6350.2 | |
| Natriumdihydrogenphosphat | Carl Roth GmbH & C0.KG | K300.2 | |
| peroxide/urea | |||
| sulphuric acid | Carl Roth GmbH & C0.KG | 4623.3 | |
| RPMI 1640 | Life technologies GmbH | 31870074 | |
| L-glutamine | Carl Roth GmbH & C0.KG | HN08.2 | |
| beta-mercaptoethanol | Sigma Aldrich | M6250 | |
| fetal calf serum | Invitrogen | 10270106 | |
| TC-flask 25 cm2 | Peske GmbH | 86-V025 | |
| TC-flask 75 cm2 | Peske GmbH | 86-V075 | |
| ethanol, 96% | Carl Roth GmbH & C0.KG | P075.1 | |
| cell strainer | VWR international | 734-0002 | |
| Falcon tubes 50 mL | Biochrom GmbH | P91050 | |
| PEG 8000 | Sigma Aldrich | 1546605 | |
| electroporation cuvette, 2mm | Biodeal Handelsvertretung Edelmann e.K. | EKL2,25 | |
| hypoxanthine | Sigma Aldrich | H9636-25G | |
| azaserine | Sigma Aldrich | A4142 | |
| thymidine | USB Europa GmbH | 22305 1 GM | |
| TC-plates 96 well | Biochrom GmbH | P92696 | |
| TC-plates 24 well | Biochrom GmbH | P92424 | |
| cryotubes, 1mL | Sigma Aldrich | V7384-1CS | |
| dimethylsulfoxid | Carl Roth GmbH & C0.KG | 4720.1 | |
| protein A sepharose | Sigma Aldrich | P3391-1G | |
| SDS sample loading buffer, Roti-Load 1 | Carl Roth GmbH & C0.KG | K929.1 | |
| unstained protein ladder | BioRad Laboratories | 161-0363 | |
| comassie brilliant blue R-250 | BioRad Laboratories | 161-0406 |
References
- Köhler, G., Milstein, C. Continous cultures of fused cells secreting antibody of predefined specificity. Nature. 7 (256), 495-497 (1975).
- Hanack, K., Messerschmidt, K., Listek, M., Böldicke, T. Antibodies and Selection. Protein Targeting Compounds. , 11-22 (2015).
- Walsh, G. Biopharmaceutical benchmarks. Nat Biotechnol. 32 (10), 992-1000 (2014).
- Pauly, D., Hanack, K. How to avoid pitfalls in antibody use. F1000 Res. 7 (4), 691-698 (2015).
- Bradbury, A., Plückthun, A. Reproducibility: Standardize antibodies used in research. Nature. 5 (518), 27-29 (2015).
- Polakiewicz, R. D. Antibodies: The solution is validation. Nature. 26 (518), 483 (2015).
- Baker, M. Antibody anarchy: A call to order. Nature. 26 (527), 545-551 (2015).
- Smith, G. P. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 228 (4705), 1315-1317 (1985).
- Kearney, J. F., Radbruch, A., Liesegang, B., Rajewsky, K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 123 (4), 1548-1550 (1979).
- Shulman, M., Wilde, C. D., Köhler, G. A better cell line for making hybridomas secreting specific antibodies. Nature. 16 (276), 269-270 (1978).
- Stoicheva, N. G., Hui, S. W. Electrically induced fusion of mammalian cells in the presence of polyethylene glycol. J Membr Biol. 141 (2), 177-182 (1994).
- Osterhaus, A. D., Uytdehaag, A. G. Current developments in hybridoma technology. Tijdschr Diergeneeskd. 15 (110), 835-839 (1985).
- Migneault, I., Dartiguenave, C., Bertrand, M. J., Waldron, K. C. Glutaraldehyde: behavior in aqueous solution, reaction with proteins, and application to enzyme crosslinking. Biotechniques. 37 (5), 798-802 (2004).
- Vitetta, E. S., Baur, S., Uhr, J. W. Cell Surface Immunoglobulin II. Isolation and characterization of immunoglobulin from mouse splenic lymphocytes. Journal of experimental medicine. 134, 242-264 (1971).
- Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227 (5259), 680-685 (1970).
- Browne, S. M., Al-Rubeai, M. Selection methods for high-producing mammalian cell lines. Trends Biotechnol. 25 (9), 425-432 (2007).
- Holmes, P., Al-Rubeai, M. Improved cell line development by a high throughput affinity capture surface display technique to select for high secretors. J Immunol Methods. 19 (230), 141-147 (1999).
- Weaver, J. C., McGrath, P., Adams, S. Gel microdrop technology for rapid isolation of rare and high producer cells. Nat Med. 3 (5), 583-585 (1997).
- Messerschmidt, K., Hempel, S., Holzlöhner, P., Ulrich, R. G., Wagner, D., Heilmann, K. IgA antibody production by intrarectal immunization of mice using recombinant major capsid protein of hamster polyomavirus. Eur J Microbiol Immunol. 2 (3), 231-238 (2012).
- Listek, M., Micheel, B., Hanack, K. Biomoleküle freisetzende zelle und deren selektion mittels eines oberflächenproteins. neweramabs GmbH. , (2014).

