A Free-breathing fMRI Method to Study Human Olfactory Function
Summary
We present the technical challenges and solutions for obtaining reliable functional magnetic resonance imaging (fMRI) data from the human central olfactory system. This includes special considerations in olfactory fMRI paradigm design, descriptions of fMRI data acquisition with an MRI-compatible olfactometer, odorant selection, and a special software tool for data post-processing.
Abstract
The study of human olfaction is a highly complex and valuable field with applications ranging from biomedical research to clinical evaluation. Currently, evaluation of the functions of the human central olfactory system with functional magnetic resonance imaging (fMRI) is still a challenge because of several technical difficulties. There are some significant variables to take into account when considering an effective method for mapping the function of the central olfactory system using fMRI, including proper odorant selection, the interaction between odor presentation and respiration, and potential anticipation of or habituation to odorants. An event-related, respiration-triggered olfactory fMRI technique can accurately administer odorants to stimulate the olfactory system while minimizing potential interference. It can effectively capture the precise onsets of fMRI signals in the primary olfactory cortex using our data post-processing method. The technique presented here provides an efficient and practical means for generating reliable olfactory fMRI results. Such a technique can ultimately be applied in the clinical realm as a diagnostic tool for diseases associated with olfactory degeneration, including Alzheimer's and Parkinson's disease, as we begin to further understand the complexities of the human olfactory system.
Introduction
The human olfactory system is understood to be much more than a sensory system because olfaction also plays an important role in homeostatic regulation and emotions. Clinically, the human olfactory system is known to be vulnerable to attacks of many prevalent neurological diseases and psychiatric disorders, such as Alzheimer's disease, Parkinson's disease, post-traumatic stress disorder, and depression1,2,3,4,5. Currently, functional magnetic resonance imaging (fMRI) with blood-oxygen-level-dependent (BOLD) contrast is the most valuable technique for mapping functions of the human brain. A significant amount of knowledge about specific functions of central olfactory structures (e.g., piriform cortex, orbitofrontal cortex, amygdala, and insular cortex) has been acquired with this technique6,7,8,9,10.
The application of fMRI to studies of the human central olfactory system and associated diseases, however, has been hindered by two major obstacles: rapid habituation of BOLD signal and variable modulation by respiration. In everyday life, when exposed to an odorant for a period of time, we quickly habituate to the scent. In fact, when studied using olfactory fMRI, the odor-induced fMRI signal is rapidly attenuated by habituation, which poses a challenge on stimulation paradigm designs8,10,11,12,13,14. The initial significant BOLD signal in the primary olfactory cortex only persists for several seconds after odorant onset. Therefore, olfactory fMRI paradigms should avoid prolonged or frequent odor stimulations in a short period of time. To reduce the habituation effect, some studies have attempted to present alternating odors in an fMRI paradigm. However, this approach may complicate data analysis since each odorant can be treated as an independent stimulation event.
Another technical issue arises with variability in subjects' respiration patterns; inhalation does not always synchronize with odorant administration during a fixed-timing paradigm. The onset and duration of olfactory stimulation are modulated by each individual's respiration, which confounds fMRI data quality and analysis. Some studies have attempted to mitigate this problem with visual or auditory cues to synchronize breathing and odorant onset, but the compliance of subjects is variable, especially in the clinical population. The brain activations associated with these cues could also complicate data analysis in certain applications. Thus, synchronizing inhalation with odorant delivery can be crucial for olfactory fMRI studies15.
An additional consideration vital to olfactory fMRI, especially in the data analysis process, is odorant selection. Finding an appropriate odorant concentration with respect to perceived intensity is important for quantification and comparison of activation levels in the brain under various experimental conditions or diseases. Odorant selection must also take into consideration odor valence, or pleasantness. This is known to cause divergent temporal profiles in olfactory learning16,17. Lavender odor was chosen for this demonstration partially for this reason. Depending on the purpose of a specific study, different odorants may be better choices. In addition, trigeminal stimulation must be minimized to reduce activation not directly related to olfaction18.
In this report, we demonstrate an fMRI technique to set up and run a respiration-triggered paradigm using an olfactometer in the magnetic resonance environment. We also present a post-processing tool that can diminish some timing errors that may have occurred during data acquisition in an attempt to further improve data analysis.
Protocol
The following experimental protocol followed the guidelines of the Institutional Review Board of the Pennsylvania State University College of Medicine, and the human subject gave written informed consent before participating in the study.
Note: For the purpose of demonstration, a simple odor stimulation paradigm using a commercially available, MRI-compatible olfactometer is presented. This paradigm has proven effective in reducing the habituation effect and has produced reliable olfactory fMRI data15. Certain steps outlined in this protocol may be specific to the type of olfactometer used. However, any type of equipment—home-made or commercially available with similar capabilities—may be used in an analogous fashion. The olfactometer must be capable of monitoring respiration as well as presenting a sequence of odorants with precise timing. Make sure that the whole odor delivery system (including the olfactometer) is built with materials inert to odorant chemicals (e.g., glass and polytetrafluoroethylene), and the odor pathway is smooth and air-tight with minimal dead space.
1. Paradigm Design
- Create a new paradigm by specifying the airflow valve sequence on a programmable olfactometer.
NOTE: The valve sequence is the order and timing of the opening and closing of specific air channels that hold different concentrations or types of odorants. In this demonstration, each of the valves for the six channels was opened twice for a total of twelve odor deliveries. Whenever one valve was open, all the other valves were closed, and each valve was opened again only after all the other valves had already opened once.- Assign duration for the stimulus (the opening of a specific channel) as well as duration for the channel to be closed.
NOTE: In this demonstration, the duration for the odor presentation was 6 s, while the duration for the channels to be closed varied from 22 s to 38 s. - Set the number of repetitions for the sequence of valve opens and closes. Here, the number of repetitions is 1.
- Interleave each odorant presentation with a presentation of odorless air at the same flow rate. For example, deliver the airflow to the subject with or without odor at a flow rate of 6 L/min in 50% relative humidity and room temperature at 22 °C.
NOTE: This is important, as variations in airflow may cause tactile sensation.
- Assign duration for the stimulus (the opening of a specific channel) as well as duration for the channel to be closed.
2. Odorant Preparation
- Choose a proper odorant for the odor stimulation paradigm by considering the odor valence, pleasantness, intensity, familiarity, and trigeminal component (see Table 1).
Note: Table 1 lists some commonly used odorants. Lavender odor was chosen for this demonstration because it has minimal trigeminal stimulation at low to moderate concentrations and is generally perceived as pleasant and familiar. - Choose a proper solvent (e.g., water, mineral oil, 1,2-propanediol, ethanol) to prepare the odorant solutions.
NOTE: Here, 1,2-propanediol was used as the solvent for odorant solution preparation. - Choose a proper odorant concentration for the odor stimulation paradigm. For example, dilute lavender oil in 1,2-propanediol at 0.10% (volume/volume) concentration for the olfactory stimulation19.
NOTE: This can be done by a psychophysical evaluation of a series of different concentrations by a group of normal subjects. - Put the proper odorant solutions in the odorant containers. Ensure that all containers have the same amount of space, same amount of solution, and same surface area for the solution. For example, use six 300-mL size glass bottles as the odorant containers with each bottle holding 50 mL of 0.10% lavender oil solution.
- Connect all the odorant containers to the proper channels for odor delivery.
3. Olfactometer Set-up
- Check the connections to ensure that all odorant containers are properly attached to the odorant carrier. Do not overtighten, as this can damage the seal. Proper tightness is ensured in a later step by checking the airflow through each odorant container.
- Place the odorant carrier in the magnet room and connect each tube to the olfactometer outside of the room, as the main unit is not MR-compatible. Visually check for any kinks in the tubing, as this will affect airflow. The airflow of each channel will be checked in a later step.
- Securely connect all tubes from the olfactometer to the odorant carrier by matching the numbers to the correct ports. For accuracy, color-code the tubes, such that pink for channel 1, blue for channel 2, etc.
- Ensure the air flow through all the channels is consistent by attaching a flowmeter to the output end of the tubing. Manually open each channel on the control panel of the olfactometer, adjust the total air flow as well as flow rates of each channel and the flush line until the flow rate of each channel is consistent.
- Connect the face mask or nose piece to the odorant carrier with polytetrafluoroethylene (PTFE) tubing. Make sure that the air flow (e.g., 6 L/min) delivered to the subject is consistent when the channels are switched.
- Connect the radiofrequency trigger from the MRI system to the “trigger in” port on the olfactometer to synchronize the odor stimulation paradigm and fMRI image acquisition. An optical-electrical signal converter may be needed.
- Adjust the total air flow and flow rates for each channel and the flush line to the designed amounts. For example, a total air flow of 6 L/min and the flow rates for each channel and the flush line to be 3 L/min.
- Connect the pneumatic respiratory sensor belt to the response port of the olfactometer via the pneumatic-electrical signal converter box.
- If a subjective response is needed, connect the pneumatic response pad to the response port of the olfactometer via the pneumatic-electrical signal converter box.
4. Experimental Procedure
- Conduct a pre-screening to ensure that the MRI procedure is safe for the subject.
- Ask the subject about medical history, including potential implants, claustrophobia, or other preexisting conditions that may interfere with the subject’s ability to safely participate in the fMRI study. Additionally, perform a smell threshold test of the odorants to ensure that the subject can smell the odorants during the experiment.
- Have the subject lie supine on the MRI examination bed. Place the face mask or nose piece properly on the subject to ensure air blows into the nostrils. Place the respiratory sensor on either the chest or abdomen. Ask the subject to breathe normally. Manually adjust the tightness and placement of the belt holding the respiratory sensor according to the respiration pattern seen on the olfactometer display.
- Create a data folder to record the respiratory data in the olfactometer. Click "file manager", enter the subject ID assigned to the current subject, then "confirm" the input.
- Use the "paradigm check" option to test the synchronization of odor delivery and inhalation without involving stimulus delivery, and if necessary, manually adjust the "valve delay" time to ensure the onset of odor delivery is synchronized with the subject's inhalation phase.
- Set the synchronization between odor stimulation and fMRI image acquisition by selecting “trigg-in” mode on the control unit of the olfactometer.
Note: This allows the odor stimulation paradigm to be started with an external trigger over the “trigger in” port originated from the MRI system. Thus, the paradigm will not run until the external trigger from the scanner is received. Please note what kind of trigger pulse (electrical or light) that the MRI scanner sends out. A signal converter may be needed to link the two systems. - Activate the respiratory trigger by selecting “resp trigger start” on the control unit of the olfactometer.
Note: When activated, the start of each paradigm sequence element is synchronized with inhalation. This can be achieved empirically by delaying the odor delivery approximately half of a cycle of respiration from the beginning of the exhalation phase. - Start the fMRI image acquisition on the MRI console; the odor stimulation paradigm will start as soon as image acquisition starts. Monitor the respiration pattern for any irregular respiratory activity.
Note: Irregular respiratory activity may be in the form of plateaus, wider and longer cycles, or erratic waves. Here, a BOLD signal-sensitive T2*-weighted echo-planar-imaging sequence was used for fMRI image acquisition with 2,000 ms repetition time, 30 ms echo time, 90° flip angle, 220 mm × 220 mm field of view, 80 × 80 acquisition matrix, 30 4-mm-thick axial slices, and acceleration factor of 2 for integrated parallel imaging techniques. - At the completion of the imaging protocol, move the subject out of the magnet and remove the face mask/nose piece.
5. Olfactometer Clean-up
- Power off the air pump. Detach the odorant containers from the odorant carrier and replace with clean, empty ones.
- Power on the air pump. Flush each channel with odorless air for 5 min to remove residual odorants in the air line.
- Shut down the olfactometer.
- Disinfect the nose piece or face mask with alcohol wipes. Rinse the face mask or nose piece with warm water and then air dry.
6. Data Analysis
- To process the data, load the respiration data file to the open-source software Olfactory Network Stimulation Editing Tool (ONSET) (www.pennstatehershey.org/web/nmrlab/resources/software/onset)15
Note: The ONSET software was developed by Xiaoyu Sun. Onsets of odor stimulation based on the timing of the paradigm and respiration trace will be automatically detected. The actual stimulation vector is defined as the start time of each effective inhalation during odor delivery.- Measure and compare the respiration rate and volume (the area under each inhalation and exhalation phase pair) between odor and odorless periods15.
NOTE: There should be no significant difference in these respiratory parameters between the odor and odorless periods. - Process the fMRI data with the actual onset and duration vectors from ONSET for the activation of the central olfactory system15.
- Measure and compare the respiration rate and volume (the area under each inhalation and exhalation phase pair) between odor and odorless periods15.
Representative Results
Figure 1 demonstrates the set-up of olfactory fMRI inside and outside of the magnet room, taking into account MR-compatibility. Figure 2a demonstrates a standard fixed-timing paradigm, while Figure 2b demonstrates a paradigm where the "respiration trigger" allows for the synchronization of odor delivery and inhalation.
A regular respiration pattern with clear inhalation peaks is vital to the implementation of an accurate respiration-triggered paradigm. Thus, adjustment of the respiration sensor is an important step in experiment setup. Figure 3 demonstrates sample respiration traces when the respiration sensor was set up incorrectly (Figure 3a) and correctly (Figure 3b). If the respiration pattern is irregular or the respiration signal plateaus, the olfactometer will be unable to accurately determine the respiration pattern, and the odor presentation cannot be synchronized with the subject's inhalation.
With a respiration-triggered, odor stimulation paradigm, the onset and duration vectors for the odor stimulation will vary among subjects. To analyze olfactory fMRI data, the actual onset and duration vectors can be determined with ONSET, and the fMRI data can be processed following standard procedures with these vectors. Figure 4 shows a sample brain activation map responding to respiration-triggered odor stimulation processed by open-source software SPM8 (www.fil.ion.ucl.ac.uk/spm) with actual odor onset and duration vectors following standard processing procedures. Significant odor-related activation was detected in the bilateral primary olfactory cortex, right insular cortex, right supramarginal/angular gyrus, left caudate nucleus, and left postcentral/supramarginal gyrus (family-wise error corrected, p < 0.05, extent threshold = 6 voxels).
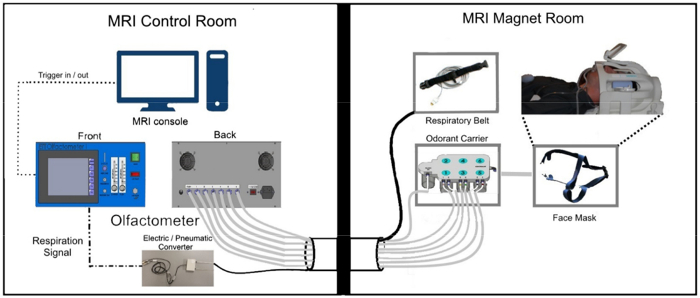
Figure 1: Schematic diagram for experimental set-up. MRI-compatible elements placed in the magnet room are connected to the MRI console and olfactometer box housed in the control room through a penetration panel with a waveguide in the wall that separates the two rooms. Please click here to view a larger version of this figure.
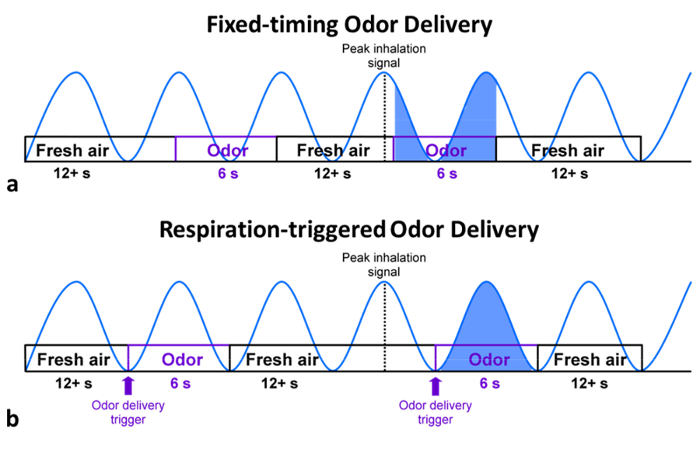
Figure 2: Illustration of odor delivery paradigms. (a) Fixed-timing paradigms, frequent problems in synchronization between respiration and odor delivery lead to variability in the amount and onset of odor delivery in the fixed-timing paradigm. (b) Respiration-triggered paradigms tend to produce more consistent results across subjects. Please click here to view a larger version of this figure.
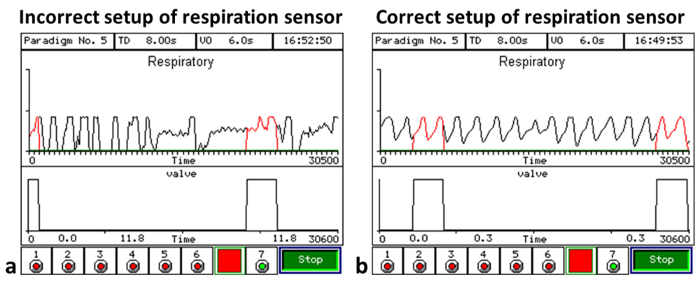
Figure 3: Sample respiratory diagrams. (a) An example respiration trace when the respiration sensor is not set up properly; the respiration patterns plateau and become irregular. (b) A representative regular respiration pattern recorded with a correctly placed respiration sensor; in this case, the respiration patterns are consistent with level peaks, and odor delivery can be synchronized with inhalation. Please click here to view a larger version of this figure.
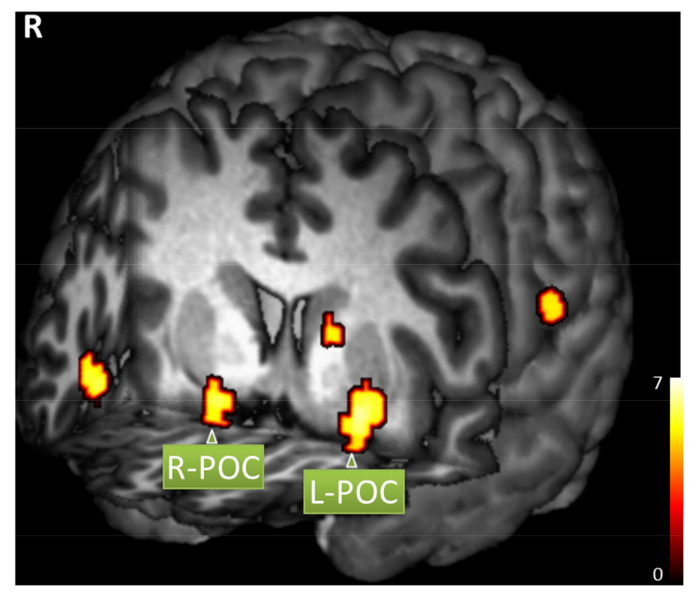
Figure 4: Sample brain activation map. A healthy subject responded to respiration-triggered lavender odor simulation (family-wise error corrected, p < 0.05, extent threshold = 6 voxels). Significant activation includes right primary olfactory cortex (POC, MNI coordinates x = 20, y = 6, z = -14), left POC (x = -22, y = 4, z = -10), right insular cortex (x = 46, y = 20, z = -10), right supramarginal/angular gyrus (x = 66, y = -48, z = 28), left caudate nucleus (x = -14, y = 6, z = 10), and left postcentral/supramarginal gyrus (x = -66, y = -24, z = 20). Please click here to view a larger version of this figure.
| Odorant | Smells like | Compound | Trigeminal stimulation | Solvent |
| Acetaldehyde29 | Green, sweet | Simple | No | Water |
| Ammonia29 | Pungent, cleaner | Simple | Yes | Water |
| Amyl Acetate30 | Banana, apple | Simple | Some | Water |
| n-Butanol31,32 | Mildly alcoholic | Simple | No | Water |
| n-Butyl Acetate31 | Sweet and fruity | Simple | Yes | Water |
| Butyric Acid33 | Sour, rancid | Simple | Yes | Water |
| Citral30,33 | Lemon | Simple | Some | Water |
| Carbon Dioxide34,35 | Odorless | Simple | Yes | N/A |
| Ethyl Butyrate30 | Pineapple | Simple | Yes | Water |
| Eucalyptol35 | Eucalyptus | Simple | Yes | Ethanol |
| Eugenol33,36 | Clove, spicy | Simple | No | Ethanol |
| Geraniol33 | Sweet rose, floral | Simple | No | Ethanol |
| Hydrosulfuric Acid34,36 | Rotten eggs | Simple | No | Water |
| Lavender24,37 | Lavender | Complex | No | Ethanol |
| Menthol33 | Peppermint | Simple | Yes | Ethanol |
| Methyl Salicylate33 | Wintergreen mint | Simple | Yes | Ethanol |
| Patchouli38 | Wet soil | Complex | Yes | Ethanol |
| 1-Propanol31 | Rubbing alcohol | Simple | Yes | Ethanol |
| Phenethyl Alcohol36,39 | Rose | Simple | No | Ethanol |
| Rosemary Oil38 | Rosemary | Complex | Yes | Ethanol |
| Sulfur Dioxide29 | Irritating, pungent | Simple | Yes | Water |
| Valeric Acid33 | Rancid cheese | Simple | Yes | Water |
| Vanillin29 | Vanilla | Simple | No | Ethanol |
| Ylang Ylang38 | Floral perfume | Complex | Yes | Ethanol |
| ***See end of manuscript for references | ||||
Table 1: Common odorants used in olfactory fMRI studies.
Discussion
Experimental procedures should be considered carefully and executed properly for collection of reliable olfactory activation data. The critical steps within the protocol include implementing a respiration-triggered paradigm to synchronize odor delivery with image acquisition, preparing proper concentrations of odorants to control psychophysical responses, setting up the olfactometer with reliable stable respiration signal and constant air flow, and post-processing respiration and odor administration timing data using ONSET to retrospectively adjust the odor onset vectors. Confounding variables such as habituation, psychophysical response, and respiration patterns need to be taken into account when designing a paradigm and analyzing data. When a subject is exposed to prolonged odor, activation of the primary olfactory cortex decreases within seconds of exposure, which makes it necessary to utilize an event-related paradigm with a sequence of brief administrations of odorants. Sniffing also should be monitored closely as it could induce activation in the piriform cortex even without an odor8. Most importantly, respiration is a major confounding variable if it is not synchronized with odorant administration. We have shown that the synchronization of inhalation and odor onset with a respiration-triggered paradigm produces more reliable activation15.
The most common issue with the free-breathing fMRI method is the poor synchronization between the event of odor delivery and inhalation, which can be caused by three imperfections in experimental setup. First, and most commonly, the respiration sensor is not set up properly. When the chest belt is too tight, the respiration signal will plateau, which will cause poor synchronization. Second, the "valve delay" time is not well calibrated, which can cause the odor delivery to be too early or too late in the respiration cycle. Third, the subject's respiration pattern is not consistent after the calibration of "valve delay" time. Thus, a pre-scan training for the subject to breathe normally in the magnet and a close monitoring of the respiration pattern during the fMRI scan are important.
It is important to consider intensity, valence, and trigeminal stimulation when selecting odorants for the study, as these variables can cause different types of psychophysical responses and associated fMRI activation. For example, a weak intensity may cause a tendency to sniff, whereas a strong intensity may cause involuntary breath holding or more rapid habituation. Odor intensity is also shown to be correlated with activation in the brain20. An alternate paradigm consisted of four concentrations of lavender presented in increasing intensity throughout the experiment, which effectively reduced habituation21. The valence of an odorant also activates different regions of the brain, which must be taken into account for data interpretation22. For example, one study demonstrated divergent temporal profiles through odor valence16. Additionally, many odorants have varying degrees of trigeminal stimulation, which should be considered.
It is important to recognize that this free-breathing paradigm is not necessarily suitable for all olfactory fMRI studies. It provides only an example for special considerations that are important for olfactory fMRI studies. It is also important to note that the experimental procedures demonstrated in this report are not specific to the olfactometer used. This equipment can be substituted with any olfactometer with similar capabilities. For example, the olfactometer must have respiration-monitoring capabilities, as well as the ability to perform a respiration-triggered paradigm with multiple odor sources. Additionally, while this experiment was presented using lavender, other odorants may be substituted by the investigator, though it is important to minimize confounding variables such as trigeminal stimulation and odorant concentration.
This free-breathing fMRI method aims to remove the preconditioning of the central olfactory system and reduce the inconsistency among repetitive events of odor stimulation. The preconditioning of the central olfactory system may vary from subject to subject, which can cause variations of activation in the primary olfactory structures. The consistency of the repetitive events, e.g., odor stimuli to trigger the activation of central olfactory system, is critical for the successful execution of event-related fMRI protocols. In addition, with the free-breathing technique, there can be no cues or tasks for the subjects during the execution of olfactory fMRI paradigms. As it requires minimal effort from the subject during functional data acquisition, it can become a valuable tool to study the olfactory deficits in some popular neurodegenerative disorders and diseases, e.g., Alzheimer’s disease.
Recent studies have used olfactory fMRI to explore the brain activation patterns in neurodegenerative disorders. Olfactory deficits in neurodegenerative disorders, particularly Alzheimer's disease and Parkinson's disease, include difficulty with odor detection, recognition, and identification3,23. However, while olfactory deficits are a distinct indicator in the earliest stages of disease onset, loss of olfactory function often goes unnoticed or is attributed to normal age-related decline1,23. Therefore, it is important to further explore the distinct activation patterns associated with olfactory dysfunction in such diseases to better diagnose them early on. In Alzheimer's disease, activation patterns are significantly reduced in the primary olfactory cortex, as well as the hippocampus and insula when compared to healthy, age-matched controls24. Additionally, researchers have found that in Parkinson's disease patients, the amygdala and thalamus show less activation than in healthy controls, while higher activation is seen in areas such as the left inferior frontal gyrus compared to controls2. Additional studies demonstrate hyperactivation in the piriform and orbitofrontal cortices in Parkinson's disease patients25. Such distinct activation patterns seem to extend beyond structural pathology, thus proving the importance of functional data acquisition in understanding and diagnosing neurodegenerative disorders and necessitating innovations in the accuracy and sensitivity of olfactory fMRI.
For this reason, further studies on the human olfactory system with fMRI may have a potential for developing a biomarker for early diagnosis for neurodegenerative diseases. In fact, studies are already progressing, including demonstration of sensitivity to activation levels between normal aging and Alzheimer's disease patients24,26. One such study showed that destruction of the neural network is often detectable even before cognitive deficits present themselves in some neurodegenerative diseases27. This further highlights the importance of olfactory fMRI investigation as a potential tool for earlier diagnosis of such diseases. Evidence also suggests the existence of large-scale olfactory network processing changes in Alzheimer's disease in addition to the changes seen in specific olfactory regions, emphasizing the importance of further exploration into the functional connectivity of olfaction28. Sensitivity of olfactory activation levels as a biomarker is dependent on sensitivity to odor stimulation and experimental reproducibility, which highlights the importance of reliability in mapping the olfactory system. Taken together, the example presented in this paper provides a glimpse of the ways in which olfactory fMRI can be effectively used to understand the complexities of the central olfactory system and the clinical importance of this understanding.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| 3T MR scanner | Siemens | Any MR scanner is acceptable. | |
| Olfactometer | Emerging Tech Trans, LLC | Any olfactometer with similar capabilities is acceptable. | |
| 6-channel odorant carrier | Emerging Tech Trans, LLC | ||
| Nosepiece/applicator | Emerging Tech Trans, LLC | ||
| PTFE tubing | Emerging Tech Trans, LLC | ||
| TTL convertor box | Emerging Tech Trans, LLC | ||
| Respiratory sensor belt | Emerging Tech Trans, LLC | ||
| Lavender oil | Givaudan Flavors Corporation | ||
| 1,2 propanediol | Sigma | P6209 | |
| ONSET | www.pennstatehershey.org/web/nmrlab/resources/software/onset | ||
| SPM8 | Wellcome Trust Center for Neuroimaging, University College London, London, UK |
References
- Doty, R. L., Reyes, P. F., Gregor, T. Presence of both odor identification and detection deficits in Alzheimer’s disease. Brain Res Bull. 18 (5), 597-600 (1987).
- Hummel, T., et al. Olfactory FMRI in patients with Parkinson’s disease. Front Integr Neurosci. 4, 125 (2010).
- Mesholam, R. I., Moberg, P. J., Mahr, R. N., Doty, R. L. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch Neurol. 55 (1), 84-90 (1998).
- Pause, B. M., Miranda, A., Göder, R., Aldenhoff, J. B., Ferstl, R. Reduced olfactory performance in patients with major depression. J Psychiatr Res. 35 (5), 271-277 (2001).
- Vasterling, J. J., Brailey, K., Sutker, P. B. Olfactory identification in combat-related posttraumatic stress disorder. J Trauma Stress. 13 (2), 241-253 (2000).
- Anderson, A. K., et al. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 6 (2), 196-202 (2003).
- Gottfried, J. A., Deichmann, R., Winston, J. S., Dolan, R. J. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 22 (24), 10819-10828 (2002).
- Sobel, N., et al. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 392 (6673), 282-286 (1998).
- Sun, X., Wang, J., Weitekamp, C. W., Yang, Q. X. A Novel Data Processing Method for Olfactory fMRI Examinations. Proc Intl Soc Mag Res Med. 18 (2010), 1161 (2010).
- Zatorre, R. J., Jones-Gotman, M., Evans, A. C., Meyer, E. Functional localization and lateralization of human olfactory cortex. Nature. 360 (6402), 339-340 (1992).
- Boley, J. C., Pontier, J. P., Smith, S., Fulbright, M. Facial changes in extraction and nonextraction patients. Angle Orthod. 68 (6), 539-546 (1998).
- Furman, J. M., Koizuka, I. Reorientation of poststimulus nystagmus in tilted humans. J Vestib Res. 4 (6), 421-428 (1994).
- Loevner, L. A., Yousem, D. M. Overlooked metastatic lesions of the occipital condyle: a missed case treasure trove. Radiographics. 17 (5), 1111-1121 (1997).
- Tabert, M. H., et al. Validation and optimization of statistical approaches for modeling odorant-induced fMRI signal changes in olfactory-related brain areas. Neuroimage. 34 (4), 1375-1390 (2007).
- Wang, J., Sun, X., Yang, Q. X. Methods for olfactory fMRI studies: Implication of respiration. Hum Brain Mapp. 35 (8), 3616-3624 (2014).
- Gottfried, J. A., O’Doherty, J., Dolan, R. J. Appetitive and aversive olfactory learning in humans studied using event-related functional magnetic resonance imaging. J Neurosci. 22 (24), 10829-10837 (2002).
- Popp, R., Sommer, M., Müller, J., Hajak, G. Olfactometry in fMRI studies: odor presentation using nasal continuous positive airway pressure. Acta Neurobiol Exp (Wars). 64 (2), 171-176 (2004).
- Wang, J., et al. Olfactory Habituation in the Human Brain. Proc Intl Soc Mag Res Med. 20, 2150 (2012).
- Grunfeld, R., et al. The responsiveness of fMRI signal to odor concentration). , A237-A238 (2005).
- Jia, H., et al. Functional MRI of the olfactory system in conscious dogs. PLoS One. 9 (1), e86362 (2014).
- Karunanayaka, P., et al. Networks involved in olfaction and their dynamics using independent component analysis and unified structural equation modeling. Hum Brain Mapp. 35 (5), 2055-2072 (2014).
- Royet, J. P., et al. Functional neuroanatomy of different olfactory judgments. Neuroimage. 13 (3), 506-519 (2001).
- Doty, R. L. Influence of age and age-related diseases on olfactory function. Ann N Y Acad Sci. 561, 76-86 (1989).
- Wang, J., et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 1357, 184-194 (2010).
- Moessnang, C., et al. Altered activation patterns within the olfactory network in Parkinson’s disease. Cereb Cortex. 21 (6), 1246-1253 (2011).
- Vasavada, M. M., et al. Olfactory cortex degeneration in Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 45 (3), 947-958 (2015).
- Jacobs, H. I., Radua, J., Lückmann, H. C., Sack, A. T. Meta-analysis of functional network alterations in Alzheimer’s disease: toward a network biomarker. Neurosci Biobehav Rev. 37 (5), 753-765 (2013).
- Murphy, C., Cerf-Ducastel, B., Calhoun-Haney, R., Gilbert, P. E., Ferdon, S. ERP, fMRI and functional connectivity studies of brain response to odor in normal aging and Alzheimer’s disease. Chem Senses. 30 Suppl 1, i170-i171 (2005).
- Hummel, T., Kobal, G. Differences in human evoked potentials related to olfactory or trigeminal chemosensory activation. Electroen Clin Neuro. 84 (1), 84-89 (1992).
- Cerf-Ducastel, B., Murphy, C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 986 (1-2), 39-53 (2003).
- Cain, W. S. Contribution of the trigeminal nerve to perceived odor magnitude. Ann NY Acad Sci. 237, 28-34 (1974).
- Murphy, C., Gilmore, M. M., Seery, C. S., Salmon, D. P., Lasker, B. R. Olfactory thresholds are associated with degree of dementia in Alzheimer’s disease. Neurobiol Aging. 11 (4), 465-469 (1990).
- Doty, R. L., Brugger, W. E., Jurs, P. C., Orndoff, M. A., Snyder, P. J., Lowry, L. D. Intranasal trigeminal stimulation from odorous volatiles: Psychometric responses from anosmic and normal humans. Physiol Behav. 20 (2), 175-185 (1978).
- Kobal, G., Hummel, T. Olfactory and intranasal trigeminal event-related potentials in anosmic patients. Laryngoscope. 108 (7), 1033-1035 (1998).
- Frasnelli, J., Lundström, J. N., Schöpf, V., Negoias, S., Hummel, T., Lepore, F. Dual processing streams in chemosensory perception. Front Hum Neurosci. 6, (2012).
- Yousem, D. M., et al. Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. 818 (2), 480-487 (1999).
- Koulivand, P. H., Ghadiri, M. K., Gorji, A. Lavender and the nervous system. Evid Based Compl Alt Med. 2013, (2013).
- Yousem, D. M., et al. Functional MR imaging during odor stimulation: Preliminary data. Neuroradiology. 204 (3), 833-838 (1997).
- Hummel, T., Doty, R. L., Yousem, D. M. Functional MRI of intranasal chemosensory trigeminal activation. Chem Senses. 30 (suppl. 1), i205-i206 (2005).

