Assessing Spatial Learning and Memory in Small Squamate Reptiles
Summary
This paper describes a modification of the Barnes maze, a standard rodent paradigm used to assess spatial memory and learning, for use in small squamate reptiles.
Abstract
Clinical research has leveraged a variety of paradigms to assess cognitive decline, commonly targeting spatial learning and memory abilities. However, interest in the cognitive processes of nonmodel species, typically within an ecological context, has also become an emerging field of study. In particular, interest in the cognitive processes in reptiles is growing although experimental studies on reptilian cognition are sparse. The few reptilian studies that have experimentally tested for spatial learning and memory have used rodent paradigms modified for use in reptiles. However, ecologically important aspects of the physiology and behavior of this taxonomic group must be taken into account when testing for spatially based cognition. Here, we describe modifications of the dry land Barnes maze and associated testing protocol that can improve performance when probing for spatial learning and memory ability in small squamate reptiles. The described paradigm and procedures were successfully used with male side-blotched lizards (Uta stansburiana), demonstrating that spatial learning and memory can be assessed in this taxonomic group with an ecologically relevant apparatus and protocol.
Introduction
Many neurodegenerative diseases such as Alzheimer's present with a progressive decline in cognitive ability, typically concurrent with degradation of the brain1-4. To test for the influence of brain injury and degradation on cognitive processes, clinical research has leveraged the advantages of model rodent species and standardization of testing apparatus and protocol. In particular, spatial learning and memory processes have been assessed via several standard paradigms such as the Morris water maze, Barnes maze, and radial arm maze (for a comprehensive review of these and other paradigms, see 5,6). The rich history of these spatial learning and memory paradigms has proven quite successful, allowing researchers to understand many of the facets and nuances of the relationship among human memory, brain function, and disease.
While assessment of cognitive processes has been examined in clinical research for quite some time, research directed towards the cognitive abilities of nonmodel species is relatively new. Researchers studying cognition in nonmodel species are typically interested in the ecological and evolutionary relevance of cognitive processes, particularly in the context of survival and reproduction. Some studies in reptiles have suggested that advanced cognitive abilities, in particular spatial memory, may underlie some behaviors, particularly those concerning navigation and orientation. However, while many studies have demonstrated that reptiles can reorient after displacement7,8, the cognitive mechanisms underlying reorientation behavior have not yet been teased apart. Because of this, some studies have attempted to experimentally assess the importance of spatial learning and memory during navigation9-17. The methodology in these studies are predominantly modeled after rodent paradigms and protocols, sometimes modified for use in reptiles, but these studies have had variable success in assessing spatial memory. Some studies have demonstrated spatial learning and memory in some species11-17 while other studies found no evidence of such9,10. Thus, the role or existence of spatial learning and memory during navigation in reptiles is still unclear.
One issue that may be problematic when experimentally assessing spatial learning and memory in reptiles is the ecological relevance of the task. Reptiles are a special taxonomic group quite distinct from rodents, demonstrating large variation in ecology, behavior, and physiology. Differences in behavior across reptilian species could possibly impact assessment of spatial cognitive abilities, particularly if the paradigm used does not tap into a natural behavior. For instance, in a species that typically seeks refuge in small crevices, spatial abilities may be easily assessed using a Barnes maze whereas this maze may not be the ideal paradigm choice in a species that typically remains motionless. Similarly, most squamate reptiles are not aquatic and thus the Morris water maze may not be a suitable choice for testing spatial learning and memory (but see15); however, this maze may be an ideal choice for testing spatial abilities in turtles16. Finally, the physiology of this group must be accounted for, as reptiles are ectothermic and proper temperature maintenance, particularly of the substrate, must be considered during the testing procedure.
The protocol and paradigm presented here were used to probe for spatial learning and memory in adult side-blotched lizards (Uta stansburiana)13, a small lizard that typically flees from predators into small crevices in rocks18. Knowing this aspect of the natural history and behavior of the species, we used a modification of the traditional Barnes maze to test for spatial learning and memory. The Barnes maze is a dry-land maze and typically used for testing spatial cognition in rodent models. We modified our maze in several ways from the rodent maze, in both design and protocol (described below). Our maze consisted of a circular platform with 10 holes equidistant from each other along the perimeter of the platform (Figure 1). The protocol described here involves a subject participating in training trials to learn the location of a goal hole, then, once the subject learns the location of the goal hole, a probe trial is used to ascertain spatial memory use during navigation to the goal.
Protocol
All procedures were approved by the Penn State University Institutional Animal Care and Use Committee (IACUC – Protocol ID: 43242) and adhered to all local, state, and federal regulations.
1. Preparation
- Purchase or construct the Barnes maze, assuring that goal holes are appropriately sized for the species of interest. For this protocol, use seven adult side-blotched lizards (Uta stansburiana). Determine adequate sample size for studies using other species.
- If constructing the Barnes maze, purchase any standard, nonporous round table (106 cm in diameter), elevated 76.2 cm off the floor.
- Using a tape measure, mark 10 equidistant points spaced 26 cm apart around the perimeter of the table and 6.35 cm from the edge of the table. Use 10 holes whereas rodent mazes typically have more holes. Doing so allows for a reduction in the number of escape options.
- Using a hole saw bit (2.54 cm in diameter), drill out all 10 goal holes and sand any sharp edges.
- Mount four shelf support pegs under each hole with screws, using a home enclosure as a guide for dimensions.
- Place the maze in a quiet room with bright overhead lighting and spatial cues available for navigation (doors, cabinets, etc.).
- Mount an auto focus, wide-angle lens webcam (11.3 cm x 3.99 cm) or other suitable camera directly above the maze. Be certain that the field of view from the video feed encompasses the entire maze.
- Place the computer and observer chair at least 1 m from the maze.
- Mark the legs of the maze on the floor with a permanent marker or tape. This allows for correction of any minute movement of the maze over the course of the trials.
- Randomly assign one of the holes as "1" and number the other holes consecutively through "10". With a permanent marker, write numbers along the side of the outside of the maze for ease of reference. Ensure that the numbers are not visible to an animal within the maze.
- Randomly assign goal holes to the subjects using a random number generator.
2. Training Trials
NOTE: Reptiles have a preferred body temperature that can be obtained from the literature. If animals become sluggish over multiple training trials, the maze may be too cool and this may affect behavior. A small space heater or heat tape on the underside of the maze can adequately increase maze surface temperature, the best thermal indication of body temperature, to maintain optimum body temperature and behavioral performance.
- If subjects are housed outside of the testing room, bring animals in their home enclosures into the room at least 30 min before testing. Be certain to provide heat in home enclosures (e.g., heat lamps, heat tape) in order to maintain body temperature before testing.
- Open tracking software and webcam application for the video feed. The custom tracking software was written for Matlab and the Image Processing toolbox; other tracking software is commercially available.
- Evaluate the field of view from the video feed. If the maze is not in the field of view, check that the maze is within the marks on the floor.
- Open the worksheet containing information as to the date, start/end time of training trials, subject ID, assigned goal hole, number of training trials run, notes, and observer's initials.
- Randomize the order in which subjects will be tested. Test subjects in a systematic manner.
- Remove the first subject from its home cage and gently place the subject in the middle of the maze. Place a plastic tub over the animal; doing so prevents unconscious orientation bias by the researcher. Allow 30 s to acclimate.
- Mount the animal's home cage under the maze, directly below the assigned goal hole, by sliding the top of the enclosure into the peg mounts. Be certain that the basking rock in the home cage is directly under the hole, which will serve as a perch when the animal descends into the hole. At this point, the use of thermal or chemical cues of the home enclosure for use in navigation is irrelevant.
- Rodent mazes use a clean enclosure as an escape for the subject. However, encourage entry into the hole during training trials by mounting the animal's home enclosure under the maze.
- Gently remove the plastic tub from the top of the maze.
- Start recording of the training trial with the webcam application.
- Allow the subject to explore the maze for 10 min. If anything atypical occurs, note this in the worksheet.
- If the subject falls or runs off the table, gently return the subject to the middle of the maze. Continue timing and recording if the animal is out of the maze for 30 s or less. If the subject is out of the maze for more than 30 s, abort the trial. Record this information in the notes section of the workbook.
- If the subject descends into the goal hole unassisted, consider the trial as over at that time.
- If the subject does not descend into the goal hole, consider the trial as over after 10 min have elapsed.
- If the subject does not descend into the goal hole after 10 min, by hand gently guide the animal, head first, into the appropriate goal hole.
- Stop video and tracking software. Save video footage.
- Allow the subject to rest for at least 2 min in its home enclosure while the enclosure is still mounted under the maze.
- Using a spray bottle and paper towels, clean the top of the maze with a diluted soap mixture of 1:10 soap to water. Doing so precludes the use of chemical cues when navigating the maze. Some species, particularly those that make heavy use of chemosensory information, necessitate additional rinsing of the maze with hot water to eliminate all scent cues.
- Remove the enclosure from under the maze and either repeat the training trial with the same individual or a new individual. Each subject can be run in up to 5 training trials per day, with 30 min interval between an individual's trials.
- Repeat this procedure until a subject descends into its goal hole unassisted, in 3 different training trials, indicating that learning of the goal location has occurred. The three training trials do not need to be consecutive. If a subject reaches this criterion, the subject moves to the probe trial in order to assess spatial memory.
3. Probe Trials
NOTE: Once a subject reaches criterion, the subject has learned how to navigate to the goal. However, at this point, it is still unclear if the subject is navigating using a spatial strategy or some other navigational strategy. Probe trials test for this and should be performed the day after the subject reaches criterion in the training trials.
- Rotate the maze 180°, assuring that the legs of the maze are within the marks on the floor. Rotation of the maze allows for local cues that directly identify the goal to be in direct conflict with the more stable spatial cues.
- Repeat steps 2.1 – 2.5.2, omitting step 2.3 of mounting the subject's home enclosure under the maze. Doing so precludes the use of olfactory or other cues emanating from the home enclosure during maze exploration.
- During probe trials, subjects are free to explore the maze for the full 10 min to assess exploratory behavior. After 10 min, stop video and tracking software.
- Remove the subject from the maze and return it to its home cage.
- Clean the top of the maze with the diluted soap mixture.
4. Behavioral Measures
NOTE: For training trials, include behavioral measures such as latency to arrive at the goal hole, number of errors made (investigation of a nongoal hole; the animal's snout must be within 1 cm of the hole), and proportion of time spent in the correct quadrant of the maze.
- For training trials, determine a search strategy. A direct strategy is defined as descending into the goal hole with fewer than 3 errors. A serial strategy is examination of 3 or more consecutive maze holes along the perimeter of the maze. A random strategy is non-serial examination of maze holes with 3 or more errors.
NOTE: For probe trials, behavioral measures include latency to arrive at the spatially correct hole, number of errors made before investigating the spatially correct hole, and the proportion of time spent in the spatially correct quadrant of the maze. - For a more stringent assessment of non-random choice of the spatially correct goal hole, employ sampling without replacement when calculating chance rate.
Representative Results
This protocol allows for the experimental assessment of spatially based navigation in small lizards. A previous study successfully used this protocol to probe for spatial navigation in male side-blotched lizards13. In that particular study, males were trained to navigate to a goal hole and, once criterion was reached, progressed into a probe trial to assess the cues prioritized when navigating to a goal hole.
The representative results from that study, presented here, demonstrate that while the lizards did learn the location of the goal, the number of training trials needed to reach criterion for each individual was quite variable and ranged from 17 – 81 training trials (average: 48.29). Because of the variable number of training trials to reach criterion across individuals, trials were divided into quartiles and averaged. Over multiple training trials individuals took less time to find the goal hole, particularly between the first and fourth quartile of training trials, indicating that subjects were learning the location of the goal hole (p = 0.012, Figure 2). During the probe trials, the maze was rotated thus conflicting local and spatial cues. Individuals went to the spatially correct goal hole at a rate well better than chance (p < 0.001, Figure 3) and spent a majority of time in the spatially correct quadrant of the maze (p < 0.001), indicating that spatial cues were being prioritized during navigation.
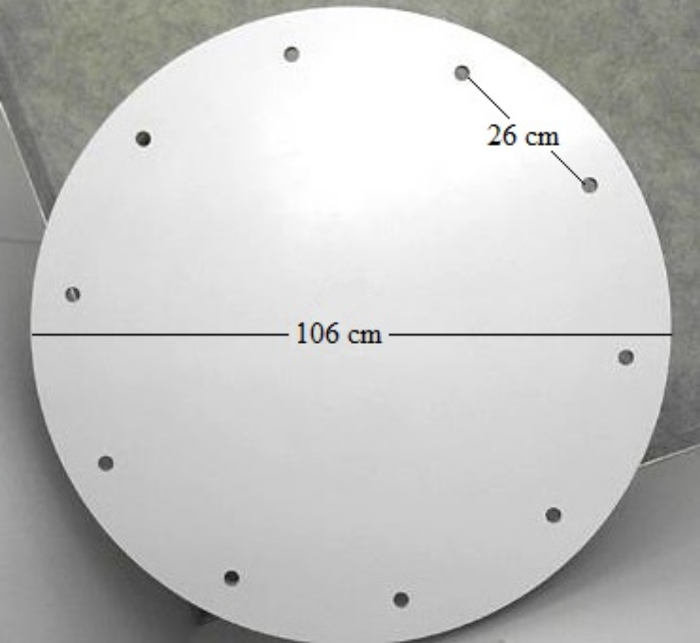
Figure 1. Barnes Maze Design for Small Lizards. A Barnes maze (diameter of 106 cm), elevated 76.2 cm off the floor. Ten equidistant goal holes are located along the perimeter and spaced 26 cm apart. Goal holes are 2.54 cm in diameter to allow small lizards to descend into the home cage mounted under the table. Please click here to view a larger version of this figure.
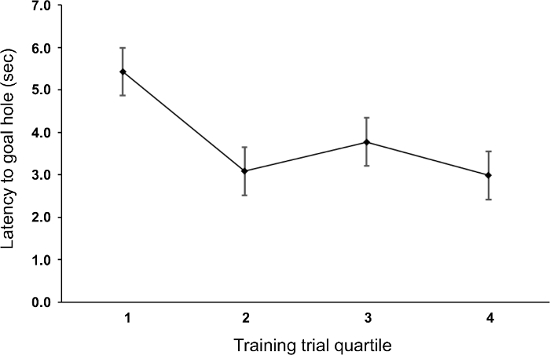
Figure 2. Training Trials. Latency (s) ±SEM to find the goal hole during training trials (n=7 individuals per quartile). Because of the variable number of training trials to reach criterion across individuals, trials were divided into quartile learning blocks and averaged. Individuals demonstrated a decrease in latency to find the goal hole, most noticeably between the first and fourth quartile of training trials (p = 0.012), indicating that individuals were learning the location of the goal hole. Data from LaDage et al. 2012. Please click here to view a larger version of this figure.
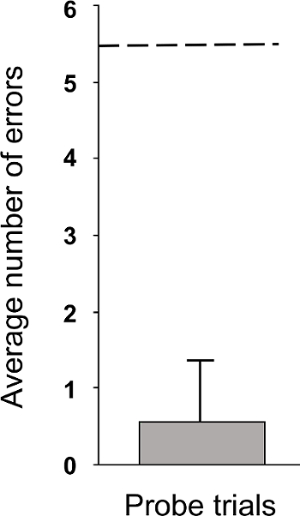
Figure 3. Probe Trials. The number of errors before choosing the spatially correct goal hole + SEM (n = 7). Individuals nonrandomly chose the spatially correct goal hole during probe trials (p <0.001), indicating a spatial strategy was used during navigation. The dashed line represents chance performance. Data from LaDage et al. 2012. Please click here to view a larger version of this figure.
Discussion
When experimentally testing for spatial learning and memory, there are several important conceptual issues that are addressed in some of the major steps in the protocol. First, subjects must demonstrate that they are learning the location of the goal hole over the course of the training trials. Attaining the preset criterion demonstrates that learning of the goal hole location has occurred. If subjects do not learn the location of the goal hole, there is no feasible way to then determine a navigational strategy. If animals do reach criterion, the probe trial is important for determining which cues are prioritized during navigation on the maze. During the probe trial, local and spatial cues are in conflict which forces a subject to prioritize the use of only one of cue for navigation. Thus, the probe trial is important for the assessment of which type of cue is being recalled and prioritized during maze navigation. Because of the focus on spatial vs. local cues, it is important to eliminate other potential cues that could be used for navigation; these cues could become a potential confound in the study. In squamate reptiles in particular, olfactory cues play a major role in mate choice, predatory avoidance, and location of food items19. Consequently, eliminating olfactory cues between trials is important to preclude following of olfactory cues while in the maze.
Although this protocol worked well with adult male side-blotched lizards13, modifications to the maze and protocol likely need to occur when testing spatial learning and memory in younger lizards or in different species (LaDage, unpublished data). For instance, juvenile lizards (i.e. younger than 9 months for side-blotched lizards), species that jump, or arboreal species may tend to flee off the maze, with little regard to descent into the goal holes. In some cases, a wall around the maze or an enclosed maze may aid in circumventing this issue, so long as the spatial cues are still visible from the surface of the maze. Also, the ecological relevance of the task must be taken into consideration when modifying any type of maze. Side-blotched lizards, which flee into crevices in rocks and into small holes in the ground, perform well on a Barnes maze. However, species that tend to remain motionless may not perform well on the Barnes maze task unless exposed to a motivator (e.g., simulated predation attempt) to induce maze exploration. Similarly, other modifications could be made to improve performance on the task including orienting the maze vertically for arboreal species, increasing the diameter of the holes for larger species, and using hides instead of holes for species that do not descend into holes. Previous studies have demonstrated that altering maze attributes can create variation in performance20-24; thus, when making modifications to the Barnes maze, differences in natural history and behavior of the species of interest must be taken into consideration.
While this protocol worked sufficiently well for probing spatial learning and memory in side-blotched lizards, there were some limitations and difficulties executing the protocol. Primarily, side-blotched lizards necessitated a large number of training trials to reach criterion, likely because there was no motivator introduced that would encourage exploration and escape from the maze. Because of this, the protocol as written is quite time-intensive, particularly when compared with the performance of rodents during training trials. Introducing aversive stimuli to increase motivation to escape the maze may circumvent this issue and decrease the number of training trials necessary to reach criterion. Additionally, because of the paucity of studies experimentally testing for spatial memory and learning in reptiles, this protocol and maze modification may not be appropriate or applicable for all species. Modifications such as the ones suggested above may help in designing an appropriate maze and protocol for the species of interest.
The protocol described here allows for experimental testing of spatial learning and memory in a small lizard. While reptile orientation and navigation have been demonstrated in the field7,8,25, little is known as to which cues are used and prioritized during navigation. The few studies experimentally testing for cue prioritization during navigation in reptiles have used varying paradigms and species which tends to preclude generalizability about spatial learning and memory across this taxonomic group. However, standardizing the testing apparatus and protocol, at least within a species and across studies, will aid in probing for generalizations in spatial learning and memory. Once this foundation has been established, other, more detailed applications of the protocol would be appropriate. For instance, introducing more obvious local cues or decreasing the reliability of spatial cues during training may induce a greater dependence on utilizing local cues for navigation within the maze. Also, the sexes may prioritize cues differently, thus assessing females on this task may also illuminate interesting intersexual differences in cue prioritization. Modifications such as these may reveal how the saliency and stability of both spatial and local cues can modulate cue prioritization in memory and subsequent recall and use of those cues during navigation.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank M. Forney, R. Maged, and K. Hellwinkle for data collection and two anonymous reviewers for comments on a previous version of this manuscript. This research was supported by an NSF award to LDL (IOS-0918268).
Materials
| Barnes maze | TSE Systems | 302050-BM/M | Available from other vendors. Alternatively, a Barnes maze can be constructed from a standard, non-porous round table. |
| Heat tape | Big Apple Pet Supply | May also use a small space heater situated on the floor under the maze. | |
| Pet keeper for small animals | Petco | 1230204 | Housing enclosure that can be mounted under the maze. |
| Nickel plated shelf support pegs | Newegg | 241941 | Pegs attached to underside of maze. Secures enclosure to maze during trials. |
| LifeCam Studio webcam | Microsoft | Q2F-00013 | Available from other vendors. Other brands of webcams may also be used. |
| Tracking software | Code custom written for Matlab and the Image Toolbox |
Video tracking software. Other tracking software such as VideoMot 2 from TSE Systems can be used. |
References
- Adriano, F., Caltagirone, C., Spalletta, G. Hippocampal volume reduction in first-episode and chronic schizophrenia: A review and meta-analysis. Neuroscientist. 18, 180-200 (2012).
- Karl, A., Schaefer, M., Malta, L. S., Dorfel, D., Rohleder, N., Werner, A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev. 30, 1004-1031 (2006).
- Shi, F., Liu, B., Zhou, Y., Yu, C., Jiang, T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer’s disease: Meta-analyses of MRI studies. Hippocampus. 19, 1055-1064 (2009).
- Videbech, P., Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry. 161, 1957-1966 (2004).
- Sharma, S., Rakoczy, S., Brown-Borg, H. Assessment of spatial memory in mice. Life Sci. 87, 521-536 (2010).
- Vorhees, C. V., Williams, M. T. Assessing spatial learning and memory in rodents. ILAR J. 55, 310-332 (2014).
- Jenssen, T. A. Spatial awareness by the lizard Anolis Cristatellus: Why should a non-ranging species demonstrate homing behavior. Herpetologica. 58, 364-371 (2002).
- Pittman, S. E., Hart, K. M., Cherkiss, M. S., Snow, R. W., Fujisaki, I., Smith, B. J., Mazzotti, F. J., Dorcas, M. E. Homing of invasive Burmese pythons in South Florida: evidence for map and compass senses in snakes. Biol. Lett. 10, (2014).
- Day, L. B., Crews, C., Wilczynski, W. Spatial and reversal learning in congeneric lizards with different foraging strategies. Anim. Behav. 57, 393-407 (1999).
- Day, L. B., Crews, C., Wilczynski, W. Effects of medial and dorsal cortex lesions on spatial memory in lizards. Behav. Brain Res. 118, 27-42 (2001).
- Holtzman, D. A. From Slither to Hither: Orientation and Spatial Learning in Snakes. Integr. Biol. 1, 81-89 (1998).
- Holtzman, D. A., Harris, T. W., Aranguren, G., Bostock, E. Spatial learning of an escape task by young corn snakes, Elaphe guttata guttata. Anim. Behav. 57, 51-60 (1999).
- LaDage, L. D., Roth, T. C., Cerjanic, A. M., Sinervo, B., Pravosudov, V. V. Spatial memory: are lizards really deficient. Biol. Lett. 8, 939-941 (2012).
- Nobel, D. W. A., Carazo, P., Whiting, M. J. Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol. Lett. 8, 946-948 (2012).
- Foà, A., Basaglia, F., Beltrami, G., Carnacina, M., Moretto, E., Bertolucci, C. Orientation of lizards in a Morris water-maze: roles of the sun compass and the parietal eye. J. Exp. Biol. 212, 2918-2924 (2009).
- López, J. C., Vargas, J. P., Gómez, Y., Salas, C. Spatial and non-spatial learning in turtles: the role of medial cortex. Behav. Brain Res. 143, 109-120 (2003).
- Petrillo, M., Ritter, C. A., Powers, A. S. A role for acetylcholine in spatial memory in turtles. Physiol. Behav. 56, 135-141 (1994).
- Zani, P. A., Jones, T. D., Neuhaus, R. A., Milgrom, J. E. Effect of refuge distance on escape behavior of side-blotched lizards (Uta stansburiana). Can. J. Zool. 87, 407-414 (2009).
- Mason, R. T., Gans, C., Crews, D. Reptilian Pheromone. Hormones, Brain, and Behavior: Biology of the Reptilia. , 114-228 (1992).
- Crawley, J. N., et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 132, 107-124 (1997).
- Schellinck, H. M., Cyr, D. P., Brown, R. E. How Many Ways Can Mouse Behavioral Experiments Go Wrong? Confounding Variables in Mouse Models of Neurodegenerative Diseases and How to Control Them. Adv. Stud. Behav. 41, 255-366 (2010).
- O’Leary, T. P., Brown, R. E. The effects of apparatus design and test procedure on learning and memory performance of C57BL/6J mice on the Barnes maze. J. Neurosci. Methods. 203, 315-324 (2012).
- O’Leary, T. P., Brown, R. E. Optimization of apparatus design and behavioral measures for the assessment of visuo-spatial learning and memory of mice on the Barnes maze. Learn. Mem. 20, 85-96 (2013).
- Patil, S. S., Sunyer, B., Höger, H., Lubec, G. Evaluation of spatial memory of C57BL/6J and CD1 mice in the Barnes maze, the Multiple T-maze and in the Morris water maze. Behav. Brain. Res. 198, 58-68 (2009).
- Roth, T. C., Krochmal, A. R. The role of age-specific learning and experience for turtles navigating a changing landscape. Curr. Biol. 25, 333-337 (2015).

