Electrospinning of Photocatalytic Electrodes for Dye-sensitized Solar Cells
Summary
The overall goal of this project was to use electrospinning to fabricate a photoanode with improved performance for dye-sensitized solar cells.
Abstract
This work demonstrates a protocol to fabricate a fiber-based photoanode for dye-sensitized solar cells, consisting of a light-scattering layer made of electrospun titanium dioxide nanofibers (TiO2-NFs) on top of a blocking layer made of commercially available titanium dioxide nanoparticles (TiO2-NPs). This is achieved by first electrospinning a solution of titanium (IV) butoxide, polyvinylpyrrolidone (PVP), and glacial acetic acid in ethanol to obtain composite PVP/TiO2 nanofibers. These are then calcined at 500 °C to remove the PVP and to obtain pure anatase-phase titania nanofibers. This material is characterized using scanning electron microscopy (SEM) and powder X-ray diffraction (XRD). The photoanode is prepared by first creating a blocking layer through the deposition of a TiO2-NPs/terpineol slurry on a fluorine-doped tin oxide (FTO) glass slide using doctor blading techniques. A subsequent thermal treatment is performed at 500 °C. Then, the light-scattering layer is formed by depositing a TiO2-NFs/terpineol slurry on the same slide, using the same technique, and calcinating again at 500 °C. The performance of the photoanode is tested by fabricating a dye-sensitized solar cell and measuring its efficiency through J-V curves under a range of incident light densities, from 0.25-1 Sun.
Introduction
Dye-sensitized solar cells (DSSCs) are an interesting alternative to silicon-based solar cells1 thanks to their low cost, relatively simple manufacturing process, and ease of large-scale production. Another benefit is their potential to be incorporated into flexible substrates, a distinct advantage over silicon-based solar cells2. A typical DSSC utilizes: (1) a nanoparticulate TiO2 photoanode, sensitized with a dye, as a light-harvesting layer; (2) a Pt-coated FTO, used as a counter electrode; and (3) an electrolyte containing a redox couple, such as I–/I3–, placed between the two electrodes, working as a "hole-conducting medium."
Although DSSCs have surpassed efficiencies of 15%3, the performance of nanoparticle-based photoanodes is still still hindered by a number of limitations, including slow electron mobility4, poor absorption of low-energy photons5, and charge recombination6. The electron collection efficiency strongly depends upon the rate of electron transport through the TiO2 nanoparticle layer. If the charge diffusion is slow, the probability of recombination with I3– in the electrolyte solution increases, resulting in a loss of efficiency.
It has been shown that replacing nanoparticulate TiO2 with one-dimensional (1D) TiO2 nanoarchitectures can improve charge transport by reducing the scattering of free electrons from the grain boundaries of the interconnected TiO2 nanoparticles7. As 1D nanostructures provide a more direct pathway for charge collection, we can expect that electron transport in nanofibers (NFs) would be significantly faster than in nanoparticles8,9.
Electrospinning is one of the most commonly used methods for the fabrication of fibrous materials with sub-micron diameters10. This technique involves the use of high voltage to induce the ejection of a polymer solution jet through a spinneret. Due to bending instability, this jet is subsequently stretched many times to form continuous nanofibers. In recent years, this technique has been extensively used to fabricate polymeric and inorganic materials, which have been used for numerous and diverse applications, such as tissue engineering11, catalysis12, and as electrode materials for lithium ion batteries13 and supercapacitors14.
The use of electrospun TiO2-NFs as the scattering layer in the photoanode can increase the performance of DSSCs. However, photoanodes with nanofibrous architectures tend to have poor dye absorption due to surface-area limitations. One of the possible solutions to overcome this is to mix NFs and nanoparticles. This has been shown to result in additional scattering layers, improving light absorption and overall efficiency15.
The protocol presented in this video provides a facile method to synthesize ultralong TiO2 nanofibers through a combination of electrospinning and sol-gel techniques, followed by a calcination process. The protocol then illustrates the use of the TiO2-NFs in combination with nanoparticulate TiO2 for the fabrication of a dual-layer photoanode with enhanced light-scattering capability using doctor blading techniques, as well as the subsequent assembly of a DSSC using such a photoanode.
Protocol
1. Precursor Solution Preparation
NOTE: Please consult all relevant material safety data sheets (MSDS) before use. Several of the chemicals used in this procedure are harmful and/or toxic to humans. Nanomaterials may have additional hazards compared to their bulk counterpart. Please use the appropriate safety measures and personal protective equipment.
- Place 5 g of titanium(IV) n-butoxide, 1 g of polyvinylpyrrolidone (PVP), 1 mL of glacial acetic acid, and 10 mL of absolute ethanol into a sample vial.
- Use a magnetic stirring plate to mix the solution until it has become homogeneous and no bubbles can be observed.
2. Electrospinning and Calcination of the Nanofibers
- Prepare the needle used for the electrospinning process by cutting off the tip of a 21 G needle and sanding it down using moderate-grade sandpaper until the tip is completely flat.
- Mount the needle on a disposable 10 mL syringe.
- Load some of the precursor solution into the syringe and place it on the syringe pump.
- Wrap the collector plate in aluminum foil and place it directly in front of the needle tip.
NOTE: The distance from the needle to the plate should be 20 cm. - Connect the collector plate to the ground and the needle to the high-voltage power source.
- Place the protective shield around the setup.
- Set the flow rate on the syringe pump to 1 mL/h and begin pumping.
- As soon as some solution appears at the tip of the needle, turn on the high-voltage source and set it to 15 kV.
NOTE: At this point, fibers are going to collect on the plate. The setup be left running for as long as necessary to achieve the desired thickness of the fiber mat. - After the spinning is completed, turn off the high-voltage source and syringe pump. Remove the foil from the collector plate.
- Let the fibers rest overnight and then peel them off the aluminum foil.
- Place the peeled-off fibers in a crucible and place that into a muffle furnace.
- Calcinate the fibers by setting up a temperature ramp of 5°/min up to 500 °C and holding for 2 h to remove the PVP and to produce pure TiO2 nanofibers.
- Once the calcination process is completed, leave the furnace closed until the temperature reaches below 80 °C to avoid any thermal shock, which may damage the fibers.
3. Electrode Fabrication
- Preparation of the slurries
- Add 500 mg of titanium dioxide paste to 20 mL of ethanol in a round-bottomed flask.
- In a separate flask, mix 500 mg of the electrospun TiO2-NFs with another 20 mL of ethanol.
- Sonicate the solutions for 2 h using a bath sonicator.
- Once uniform mixtures are obtained, add 2 mL of terpineol to each flask and sonicate for another 15 min.
- Evaporate the solvent from both flasks using a rotary evaporator to obtain the slurries.
- Doctor blading and sintering
- Using a diamond glass cutter, cut an FTO-conductive glass slide into a 2 cm x 2 cm square.
- Secure the FTO slide to the work area by placing adhesive tape on the glass slide, leaving a 0.4-cm2 area exposed in the center. To avoid an irregular coating, place the tape on two parallel sides first and then on the other two.
- Deposit a few drops of the TiO2-NP slurry on the exposed center of the slide.
- Use a razor blade to spread the slurry evenly over the exposed area.
- Once a uniform coating is achieved, carefully remove the adhesive tape.
- Place the coated slide in a furnace and sinter at 500 °C for 2 h.
- Repeat steps 3.2.2-3.2.6 on the same FTO slide, this time using the TiO2-NF slurry instead of the nanoparticles, to obtain the photoanode.
4. NF Characterization
- SEM characterization
- Prepare the sample for SEM by attaching a strip of adhesive carbon tape to a microscope stub. Carefully place a small quantity of nanofibers on the tape.
- Mount the stub onto a sample holder and load it into the exchange chamber of the instrument.
- Set up the instrument conditions and parameters: set the accelerating voltage to 20 kV and the working distance to 10 mm.
- Collect images of the sample, making sure that they display the overall morphology of the material.
- XRD characterization
- Gently grind some nanofibers into a fine powder and spread them evenly on an XRD stage.
- Load the sample into the diffractometer.
- Set up the acquisition parameters: use a start angle of 10°, an end angle of 80°, and a step size of 0.015°.
- Start the acquisition of the XRD data.
5. Solar-cell Fabrication
- Treat the photoanode with an aqueous solution of TiCl4 at 75 °C for 45 min. After treatment, wash it with deionized water and dry it.
- Sensitize the photoanode by submerging it in a 0.5-mM solution of ruthenium dye N719 in absolute ethanol for 24 h under dark conditions.
- Place a sheet of sealing film on top of the sensitized photoanode to serve as a thermoplastic gasket between the photoanode and the counter-electrode.
- Place a Pt-coated FTO counter-electrode with a pre-drilled hole in the center, on top of the sealing film, such that both sides face each other.
- Heat the assembled cell to 100 °C for 15 min to seal the gasket.
- Deposit a few drops of a redox mediator, consisting of a solution of 1-propyl-3-methylimidazolium iodide (0.8 M), iodine (0.1 M), and benzimidazole (0.3 M) in 3-methoxypropionitrile, on top of the pre-drilled hole of the counter-electrode.
- Place the cell in a vacuum desiccator to let the redox mediator fill the internal space of the assembled cell.
6. J-V Curve Characterization
- Acquire the J-V curves using a digital source meter under 100 mW/cm2 illumination from a xenon-arc source passed through an AM1.5G filter.
Representative Results
The TiO2 nanofibers were characterized using SEM, X-ray photoelectron spectroscopy (XPS), and XRD. The nanostructure of the photoanode was characterized using SEM. The performance of the assembled DSSC was tested using a solar simulator and a source measure unit.
The SEM image in Figure 1A shows that the nanofibers synthesized using this protocol have a porous structure and a high aspect ratio. They are up to several micrometers in length and only a few hundred nanometers in diameter. The cross-section in Figure 1B shows three layers: the top layer is the fibrous scattering layer of the TiO2-NF, the second layer is the blocking layer of TiO2-NP paste, and the bottom layer is the FTO substrate. Both layers are approximately 7 µm, resulting in a total film thickness of approximately 14 µm.
The diffractogram in Figure 2 shows a series of peaks corresponding to the anatase phase of titanium dioxide. The sharp peaks in the spectra indicate that the nanofibers are highly crystalline, which is a favorable feature for this type of application. Figure 3 shows the Ti 2p XPS spectrum for TiO2 NF and NP photoelectrodes. TiO2 was verified by the Ti 2p peaks present at binding energies of 465 eV (Ti2p(1/2)) and 459 eV (Ti2p(3/2)).
The J-V curve in Figure 4 shows that under 1-Sun illumination (solid line), The TiO2-NF DSSC achieved a short-circuit current density (JSC) of 8.30 mA/cm2, an open circuit voltage (VOC) of 0.63 V, a fill factor (FF) of 56%, and a power conversion efficiency (PCE) of 2.90%. To investigate further, the dependence of the cell performance on illumination intensity (from 0.25-1 Sun) was measured. The characteristic values are plotted in Figure 5. The JSC increases linearly up to 0.75 Sun (Figure 5A); the slope then increases considerably between 0.75 and 1 Sun. The VOC exhibits a linear increase across the measured range (Figure 5B). In Figure 5C, the FF is stable between 0.25 and 0.75 Sun, but it rapidly decreases up to 1 Sun; this may be due to an increase in charge recombination. Figure 5D shows that, at an incident light intensity of 25 mW/cm2, the DSSC achieves a PCE of 3.7%, indicating higher performance under lower illumination intensities. As a comparison, Figure 6 shows TiO2 NP DSSCs, which achieved a JSC of 6.53 mA/cm2, a VOC of 0.63 V, an FF of 57%, and a PCE of 2.35%.
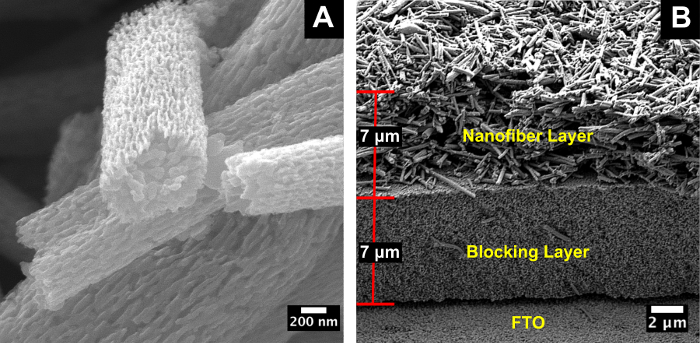
Figure 1: Images of the electrospun TiO2-NFs. (A) High-resolution image of the electrospun TiO2-NFs. (B) Cross-section SEM; the top layer is the light-scattering nanofiber layer, and the bottom layer is the blocking TiO2-NP layer. Figures adapted and reprinted with permission from Macdonald et al.16. Please click here to view a larger version of this figure.
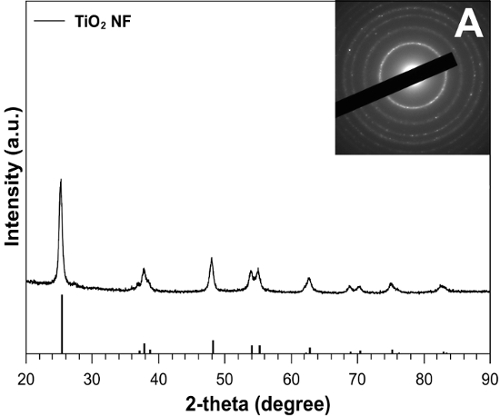
Figure 2: XRD spectrum of the electrospun TiO2-NFs. The inset shows the selective-area electron diffraction (SAED) pattern indicative of TiO2 in the anatase phase; reprinted with permission from Macdonald et al.16. Please click here to view a larger version of this figure.
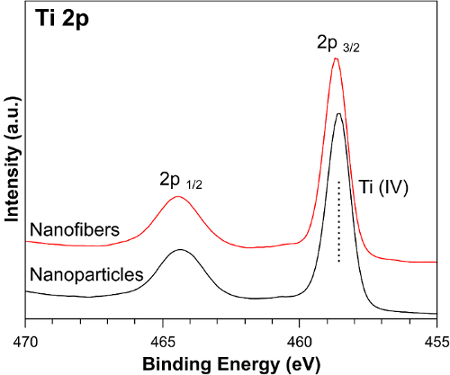
Figure 3: Ti 2p XPS spectrum for TiO2 NF and NP photoelectrodes. The solid red curve shows the spectrum for nanofibers, and the solid black curve shows the spectrum for nanoparticles. Please click here to view a larger version of this figure.
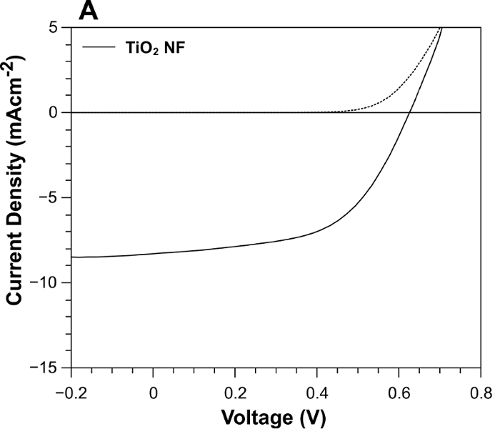
Figure 4: J-V curve under 1-Sun illumination of the DSSC made with TiO2 NFs. (A) The dark current is represented by the dotted line. Reprinted with permission from Macdonald et al.16. Please click here to view a larger version of this figure.
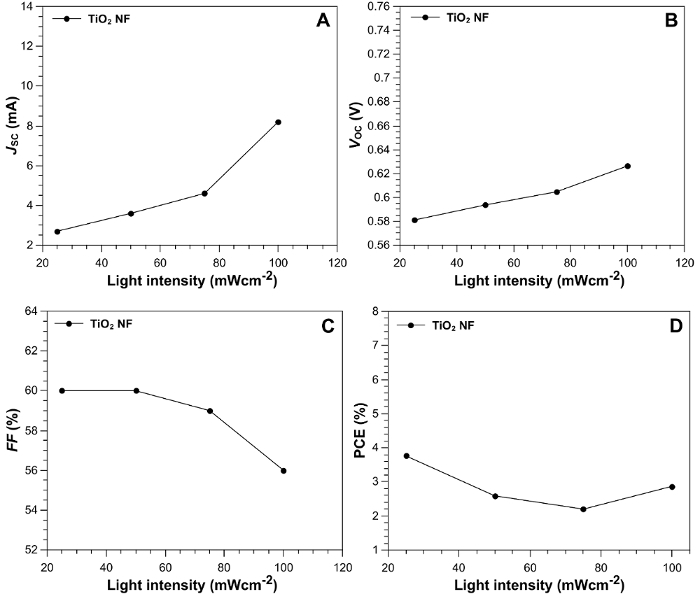
Figure 5: Device characterization parameters. (A) JSC, (B) VOC, (C) FF, and (D) PCE as a function of light intensity, from 25 mW/cm2 (0.25 Sun) to 100 mW/cm2 (1 Sun). Reprinted with permission from Macdonald et al.16. Please click here to view a larger version of this figure.
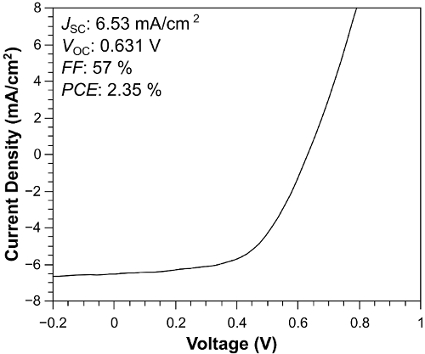
Figure 6: J-V curve under 1-Sun illumination of the DSSC made with TiO2-NPs. The curve shows TiO2 NP DSSCs, which achieved a JSC of 6.53 mA/cm2, a VOC of 0.63 V, an FF of 57%, and a PCE of 2.35%. Please click here to view a larger version of this figure.
Discussion
The methods presented in this work describe the fabrication of efficient nanofibrous photoanodes for photocatalytic devices such as DSSCs. Electrospinning is a very versatile technique for the fabrication of nanofibers, but a certain level of skill and knowledge is required to obtain materials with optimal morphologies. One of the most critical aspects to obtaining good nanofibers is the preparation of the precursor solution: there are some key factors, such as the concentration of the carrier polymer and the choice of titanium precursor, that can have a critical impact on the final structure of the material. A low concentration of carrier polymer will lead to the formation of beads or the total absence of a nanofibrous structure. On the other hand, a too-high concentration will excessively increase the viscosity of the solution and lead to an increase of the diameter of the nanofiber, with a consequent loss of surface area and charge mobility. The inorganic precursor needs to be highly soluble and should not react or decompose in the presence of the other components of the solution. It should also calcinate easily into the final material, without leaving any undesired sub-products.
The instrumental parameters (i.e., voltage, tip-to-collector distance, and needle diameter) also have an important effect on the nanofiber morphology. Although a general trend can be observed when changing these conditions using a specific precursor solution, this does not necessarily apply to other solutions, as they may be affected differently by modifications of the electric field and other instrumental conditions17.
Thanks to the versatility of this technique, a wide range of nanomaterials can be fabricated and used in several different applications, such as energy conversion and storage, catalysis, filtration, composite materials, and superhydrophobic surfaces. Furthermore, this method shows significant potential for upscaling, which is a key factor for its use in commercial applications.
The calcination process needs to be performed at a high enough temperature to completely remove the carrier polymer and to promote the crystallization of TiO2, but without disrupting the nanostructure of the material. The calcination temperature also needs to be reached at a relatively slow heating rate to avoid any thermal shock, which might damage the fibers. This also applies to the cooling process: after the heat treatment is finished, the furnace must remain closed until the temperature has reached a safe temperature (<80 °C).
Doctor blading is a simple and quick method that allows one to easily obtain thin-film substrates on flat surfaces. The key factor to obtaining a smooth and uniformly coated surface is the slurry viscosity: if too much dispersant is added to the mixture, the coating will present pores and have an uneven thickness; if too little dispersant is added, the resulting film will likely have cracks on its surface.
Once mastered, this technique can easily be used for any application that requires thin-film deposition for device fabrication.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| titanium(IV) n-butoxide | Sigma-Aldrich | 244112 | |
| Polyvinylpyrrolidone | Sigma-Aldrich | 437190 | |
| glacial acetic acid | Sigma-Aldrich | A6283 | |
| Ethanol, absolute | Fisher Scientific | E/0650DF/17 | |
| 20 mL Sample vials | (any) | (or larger volume) | |
| disposable 21G needle | (any) | ||
| P150 grit sandpaper | (any) | ||
| disposable 10mL syringe | (any) | (or larger volume) | |
| magnetic stirrer + stirring bar | (any) | ||
| PHD 2000 syringe pump | Harvard Apparatus | 71-2002 | (or any other syringe pump capable of outputting a 1mL/hr flow |
| Aluminium foil | (any) | ||
| Stainless steel collector plate | (custom built) | ||
| High Voltage Power Source | Gamma High Voltage Research, Inc | ES30P-10W | (or any other power supply capable of outputting +15 kV |
| Polycarbonate protective shield | (custom built) | ||
| Ceramic crucible | (any) | ||
| Muffle furnace | (any) | ||
| Titanium dioxide, nanopowder | Sigma-Aldrich | 718467 | |
| 50 mL 1-neck round bottom flasks | (any) | ||
| bath sonicator | (any) | ||
| Terpineol | Sigma-Aldrich | ||
| Rotary evaporator | (any) | ||
| FTO glass | Solaronix | TCO30-10/LI | |
| Adhesive tape | (any) | ||
| razor blade | (any) | ||
| SEM | JEOL | 6500F | |
| XRD | PANalytical | X'pert Pro | |
| Titanium Tetrachloride | Sigma-Aldrich | 89545 | |
| Ruthenizer 535-bisTBA | Solaronix | N719 | |
| sealing film | Dyesol | Meltonix 1170-25 | |
| Pt-coated FTO | Solaronix | TCO30-10/LI | |
| 1-propyl-3-methylimidazolium iodide | Sigma-Aldrich | 49637 | |
| Iodine | Sigma-Aldrich | 207772 | |
| benzimidazole | Sigma-Aldrich | 194123 | |
| 3-Methoxypropionitrile | Sigma-Aldrich | 65290 | |
| Digital source meter | Keithley | 2400 | |
| Solar Simulator | Abet technologies | 10500 |
References
- O’Regan, B., Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 353 (6346), 737-740 (1991).
- Lee, C. H., Chiu, W. H., Lee, K. M., Hsieh, W. F., Wu, J. M. Improved performance of flexible dye-sensitized solar cells by introducing an interfacial layer on Ti substrates. J Mat Chem. 21 (13), 5114-5119 (2011).
- Burschka, J., Pellet, N., et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature. 499 (7458), 316-319 (2013).
- Ohsaki, Y., Masaki, N., et al. Dye-sensitized TiO2 nanotube solar cells: fabrication and electronic characterization. Phys Chem Chem Phys. 7 (24), 4157-4163 (2005).
- Mor, G. K., Shankar, K., Paulose, M., Varghese, O. K., Grimes, C. A. Enhanced Photocleavage of Water Using Titania Nanotube Arrays. Nano Letters. 5 (1), 191-195 (2005).
- Feng, X., Shankar, K., Varghese, O. K., Paulose, M., Latempa, T. J., Grimes, C. A. Vertically Aligned Single Crystal TiO2 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis Details and Applications. Nano Letters. 8 (11), 3781-3786 (2008).
- Roy, P., Berger, S., Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angewandte Chemie International Edition. 50 (13), 2904-2939 (2011).
- Macdonald, T. J., Xu, J., et al. NiO Nanofibers as a Candidate for a Nanophotocathode. Nanomaterials. 4 (2), 256-266 (2014).
- Chuangchote, S., Sagawa, T., Yoshikawa, S. Efficient dye-sensitized solar cells using electrospun TiO2 nanofibers as a light harvesting layer. Appl Phys Lett. 93 (3), 033310 (2008).
- Li, D., Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel?. Adv Mat. 16 (14), 1151-1170 (2004).
- Li, W. J., Laurencin, C. T., Caterson, E. J., Tuan, R. S., Ko, F. K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J Biomed Mat Res. 60 (4), 613-621 (2002).
- Jia, H., Zhu, G., Vugrinovich, B., Kataphinan, W., Reneker, D. H., Wang, P. Enzyme-Carrying Polymeric Nanofibers Prepared via Electrospinning for Use as Unique Biocatalysts. Biotechnol Prog. 18 (5), 1027-1032 (2002).
- Mai, L., Xu, L., et al. Electrospun Ultralong Hierarchical Vanadium Oxide Nanowires with High Performance for Lithium Ion Batteries. Nano Letters. 10 (11), 4750-4755 (2010).
- Cai, J., Niu, H., et al. High-Performance Supercapacitor Electrode Materials from Cellulose-Derived Carbon Nanofibers. ACS Appl Mat Interfaces. 7 (27), 14946-14953 (2015).
- Joshi, P., Zhang, L., et al. Composite of TiO2 nanofibers and nanoparticles for dye-sensitized solar cells with significantly improved efficiency. Energ Environ Sci. 3 (10), 1507-1510 (2010).
- Macdonald, T. J., Tune, D. D., Dewi, M. R., Gibson, C. T., Shapter, J. G., Nann, T. A TiO2 Nanofiber-Carbon Nanotube-Composite Photoanode for Improved Efficiency in Dye-Sensitized Solar Cells. ChemSusChem. 8 (20), 3396-3400 (2015).
- Teo, W. E. . Electrospinning parameters and fiber control. , (2015).

