Introducing Clicker Training as a Cognitive Enrichment for Laboratory Mice
Summary
The development of new refinement strategies for laboratory mice is a challenging task that contributes towards fulfilling the 3R principle. This protocol introduces clicker training as a cognitive enrichment program for laboratory mice.
Abstract
Establishing new refinement strategies in laboratory animal science is a central goal in fulfilling the requirements of Directive 2010/63/EU. Previous research determined a profound impact of gentle handling protocols on the well-being of laboratory mice. By introducing clicker training to the keeping of mice, not only do we promote the amicable treatment of mice, but we also enable them to experience cognitive enrichment. Clicker training is a form of positive reinforcement training using a conditioned secondary reinforcer, the “click” sound of a clicker, which serves as a time bridge between the strengthened behavior and an upcoming reward. The effective implementation of the clicker training protocol with a cohort of 12 BALB/c inbred mice of each sex proved to be uncomplicated. The mice learned rather quickly when challenged with tasks of the clicker training protocol, and almost all trained mice overcame the challenges they were given (100% of female mice and 83% of male mice). This study has identified that clicker training for mice strongly correlates with reduced fear in the mice during human-mice interactions, as shown by reduced anxiety-related behaviors (e.g., defecation, vocalization, and urination) and fewer depression-like behaviors (e.g., floating). By developing a reliable protocol that can be easily integrated into the daily routine of the keeping of laboratory mice, the lifetime experience of welfare in the mice can be improved substantially.
Introduction
The development of new refinement strategies for laboratory mice is a challenging task that contributes to the fulfillment of the 3Rs (replacement, reduction, and refinement) of laboratory animal science1. Improvements in the field of refinement can further contribute to the well-being of the millions of animals that are used for experimental purposes. Therefore, intensive research is needed in the field. This is also a defined aim of Directive 2010/63 of the European Union. Directive 2010/63/EU points out that the lifetime experience of laboratory animals has to be enhanced and that "establishments shall set up habituation and training programs suitable for the animals, the procedures and length of the project" (Article 3.7)2.
Laboratory animals can experience many stressful situations while they are being kept and bred for experiments. Usually, the interaction between the laboratory mice and the responsible persons is rather limited. Therefore, a trusting relationship cannot develop. This can elicit increased anxiety and stress in reaction to handling, which is detrimental to the behavior and physiology, and therefore, the well-being, of the animals3,4,5,6. Furthermore, routinely performed laboratory procedures like general handling, restraint, and blood or tissue sampling can cause stress responses, which can be examined by measuring different parameters, such as stress hormones or behavior7,8. It has been shown that handling programs can efficiently decrease the anxiety toward the investigator in laboratory rodents9,10,11. Handling programs can therefore improve the animals' conditions and could contribute considerably to animal welfare5.
The goal of this study is to introduce positive reinforcement training for mice as a specific handling program. Positive reinforcement training is a form of operant conditioning that gives the investigator a means to shape animal behavior. When the animal performs a desired behavior, it is followed by a positive stimulus (here, a food reward). The intention is that the animal links the reward to the respective behavior. Clicker training is a form of positive reinforcement training using a conditioned secondary reinforcer, the "click" sound of a clicker, and has been proven to strengthen a specific behavior12.
More specifically, the click sound serves as a "time bridge" between the behavior and the upcoming reward13. The trainer clicks precisely when the animal performs the desired behavior, without any time delay14, and then presents the food reward. This strengthens the rewarded behavior, which will be performed with a higher frequency. Clicker training is widely used with companion animals and has made its way into laboratory animal science, where it has been successfully implemented with nonhuman primates13,15,16. As mice learn rather quickly when challenged with operant conditioning paradigms, the introduction of a second reinforcer should not overstrain their cognitive abilities5,17,18,19.
By introducing clicker training to the keeping of mice, we enable mice to experience cognitive enrichment. The design of cognitive enrichment must enable the mice to use their cognitive skills to solve problems and to gain control over their environment20,21. Several studies with different species prove the positive impact of cognitive enrichment on the welfare of captive animals22,23,24. Enhancing the ability of the animals to successfully cope with environmental challenges contributes to their well-being25,26.
In addition, if the animals experience low levels of stress during their lifetime, they are less prone to develop detrimental coping strategies when confronted with stressors that occur in biomedical research. Thus, the consistent implementation of cognitive enrichment can contribute to a homogenization of the subjects' phenotypes. This will contribute to the 3R principle of reduction, as it can reduce the number of subjects required to meet statistical requirements27.
By developing a reliable protocol that can be easily integrated into the daily routine of keeping laboratory mice, we can substantially improve their lifetime experience of welfare.
Protocol
Ethics Statement: The handling of the mice and the experimental procedures were conducted in accordance with European, national, and institutional guidelines for animal care.
NOTE: The protocol includes five days of interventions (Monday to Friday), with breaks on the weekends (Saturday and Sunday). The protocol can be easily adapted to meet specific needs.
1. Determining a Reward Suitable as a Second Reinforcer
NOTE: Use food rewards, such as vacuum-packed food or animal feed that meets food safety standards. For example, different kinds of nuts, chocolate, gummy bears, or dried fruits are suitable.
- Insert a small Petri dish with different food rewards (pieces of 0.5 cm3) into the home-cage (Figure 1).
- After 10 min, check for leftovers.
- If all rewards are eaten after 10 min, shorten the time span until a preference can be estimated.
- On days 4 and 5 (e.g., Thursday and Friday), add small amounts of the preferred ("winner") reward to the home-cage to establish an affectation of all mice in the cage to the reward.
- If the mice are not habituated to mouse tunnels, add a tunnel to the cage (at least 2 days before beginning the training, e.g., over the weekend).
- Repeat steps 1.2 – 1.4 on 3 consecutive days (e.g., Monday to Wednesday).
2. Clicker Training
- Preparations for each training session
NOTE: Every time a mouse needs to be lifted up in this protocol, lift it in a calm and gentle manner. Put a hand under the mouse to lift it up. During the first few times, the mice might be agitated, but the habituation process will be finished after several days. If this step creates difficulties, it is useful to refer to the protocol for "cupping on the open hand"28.- Prepare a cage with bedding material.
- Transfer the cage of the mouse being trained to a quiet place.
- Prepare the reward and have a timer and the clicker/target stick combination ready.
NOTE: If the mice are habituated to handling and already have a relationship of trust with the experimenter, the mice can be fed by hand. Otherwise, attach the reward to a stick or forceps in such a way that the mice can keep a distance from the experimenter. - Remove all objects from the home-cage, which now serves as the training area (e.g., mouse houses, nest-building material, etc.).
- Transfer the cage companions to a prepared cage.
- General rules that apply for all training sessions
NOTE: The following general rules apply for all sessions and will not be mentioned again throughout the protocol.- Train each mouse for 5 min. Alternate between 30-s training and 15-s break periods.
- Let the mouse gnaw on the reward no longer than a second. Then, take the stick (with the reward) out of the cage.
- Execute the following pattern of rewarding:
1 – 10 performances of behavior: reward every time.
11 – 21 performance of behavior: reward every second time.
From 22 performance of behavior on: reward every third time. - After 4 min of training, the next performance of the desired behavior is reinforced by a jackpot reward. Allow the mouse to gnaw at the food reward three times longer than before.
- Training session Day 1: "Linking the secondary reinforcer with the food reward"
- Set the timer to 5 min, press start, and add the mouse tunnel to the home-cage.
NOTE: Take advantage of the innate thigmotaxis of mice and place the tunnel next to a wall (Figure 2). For the first few sessions, this will enhance the probability of the mouse entering the tunnel. - Wait until the mouse inspects the tunnel. As soon as the mouse enters the tunnel, click and present the reward at the end of the tunnel.
- Let the mouse feed on the reward while sitting in the tunnel.
- Click continuously for 15 s while the mouse is sitting in the tunnel.
- As soon as the mouse leaves the tunnel, go back to step 3.2.
- Set the timer to 5 min, press start, and add the mouse tunnel to the home-cage.
- Training session Days 2 – 5: "Running through a tunnel"
- Set the timer to 5 min, press start, and add the mouse tunnel to the home-cage.
- As soon as the mouse enters the tunnel, click and present the reward at the end of the tunnel.
- As long as the mouse is in the tunnel, click and immediately present the reward in the same manner.
- Repeat this for the next 30 s, and then pause for 15 s to take the tunnel out of the cage.
- Add the tunnel again and click immediately when the mouse reenters the tunnel.
- Present the reward at the end of the tunnel.
- Allow the mouse to gnaw for up to 1 s.
- Take away the reward.
- As soon as the mouse starts re-entering the tunnel by itself, present the reward in front of the end of the tunnel (Figure 3).
- Training session week 2: "Following a target stick"
- Set the timer to 5 min, press start, and place the globe at the end of the target stick in the cage.
- As soon as the mouse shows interest in the globe, click and present the reward next to the globe (Figures 4 and 5).
- Reward the mouse only after it touches the globe with its nose.
- When the mouse has linked the performed action to the reward, change the position of the globe during the training session (Figure 6).
- Wait for the mouse to meet this last challenge.
- Place the globe in the cage.
- Shortly before the mouse touches the globe, slowly and carefully change the position of the globe by 1 cm.
- Click and reward if the mouse has crossed the distance and touches the globe.
- Repeat this procedure until the mouse follows reliably.
- Slowly extend the distance the mouse has to cross.
- Training session week 3: "Following the target stick to the experimenter's hand"
- Place one hand in the training cage while holding the clicker/target stick combination and the reward with the other hand.
- Set the timer to 5 min, press start, and place the globe at the end of the target stick next to the hand.
- As soon as the mouse shows interest in the globe, click and present the reward next to the globe.
- As soon as the mouse has met this challenge, move the globe several steps closer to the hand.
- Place the globe on the palm of the hand. Click and reward while the mouse is sitting on the palm of the hand (Figure 7).
Representative Results
The first and also one of the most important steps was the determination of an appropriate food reward. Therefore, the mice were offered different kinds of nuts, a sugar solution, marmalade, and different kinds of chocolate in a Petri dish (Figure 1). In our experience, the mice showed an obvious preference for white chocolate. Hence, we used white chocolate for all further training processes.
The actual training was implemented with a cohort of 12 BALB/c inbred mice of each sex. All mice were highly interested in the training. For the evaluation of training success, we checked for the proper performance of the desired behavior: "following the target stick" (Figure 4). The vast majority of the trained mice-all female mice and 83% of the male mice-followed the target stick (Figure 8). Female mice displayed a higher motivation for training in general and performed the respective behavior with a higher frequency throughout the training sessions. After completing 4 days of training, female mice followed the target stick with a mean of 64 times per 5 min, whereas male mice displayed this behavior only 50 times per 5 min. In 5 min, the task "following to the palm of the hand" was performed on average 55 times by female mice and 35 times by male mice (Figure 9).
To check whether the training had a positive effect on the well-being of the trained mice, we evaluated the tolerance to manipulations after completing the training. Therefore, we analyzed anxiety-related behaviors while the mice were singlehandedly restrained (i.e., grasping the scruff of the neck and the base of the tail with one hand) for 15 s. Spontaneous urination, defecation and vocalization were recorded. An untrained but gently handled cohort of 12 BALB/c inbred mice of each sex served as a control group. Trained mice displayed a significantly lower frequency of anxiety related behaviors than untrained mice. (Figure 10). Similar results were obtained when recording the vocalization linked to handling while performing the Morris Water Maze Test (Figures 11 and 12). The trained mice squeaked significantly less than the untrained mice (Figure 11) and the total number of squeaks per squeaking mouse was significantly reduced. To further evaluate this issue, we analyzed the floating behavior during the Morris Water Maze Test. Floating behavior during the Morris Water Maze Test is described as periods of time when the mice are not swimming, but are merely floating on the surface. This depression-related floating behavior turned out to be significantly reduced in the trained group (Figure 12).
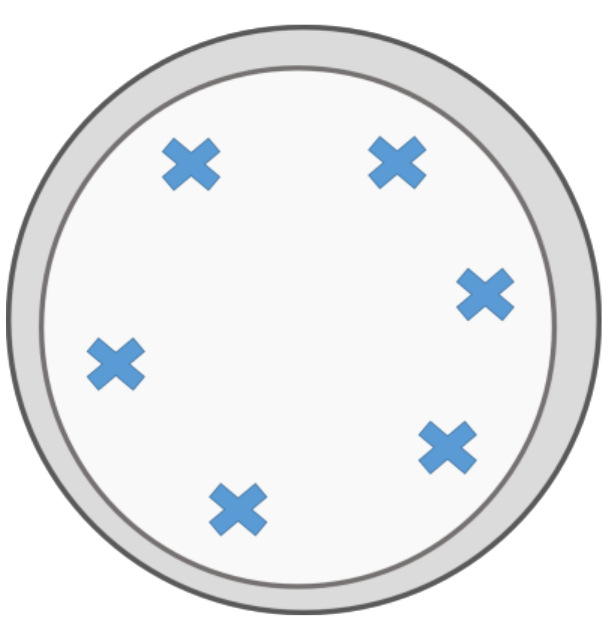
Figure 1. Presentation of Possible Rewards. Six positions for the presentation of rewards are marked in a Petri dish.

Figure 2. Position of Mouse Tunnel. To enhance the probability of the mouse entering the tunnel, the tunnel is placed next to a cage wall. Please click here to view a larger version of this figure.

Figure 3. Presenting the Reward. The appropriate position for presenting the reward outside the end of the tunnel is shown. Please click here to view a larger version of this figure.

Figure 4. Training Success. A trained mouse follows the target stick while being trained. Please click here to view a larger version of this figure.
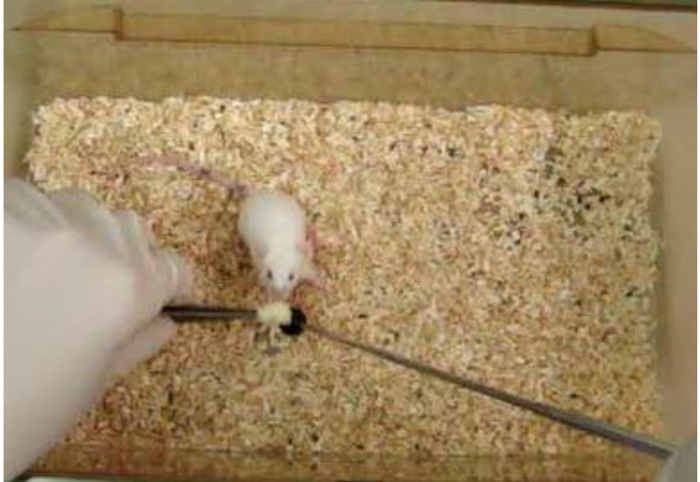
Figure 5. Rewarding. A mouse is rewarded next to the target stick during the training session. Please click here to view a larger version of this figure.
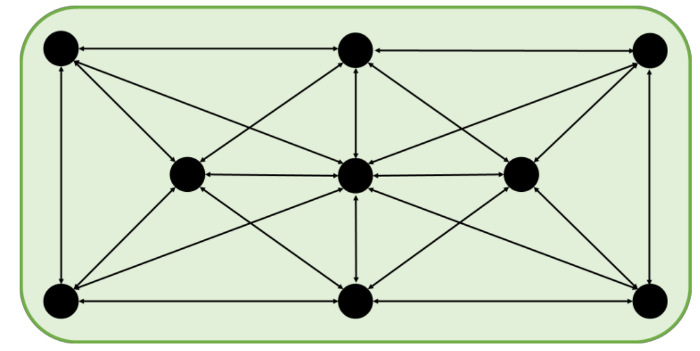
Figure 6. Alternating Target Stick Positions in the Second Week of Training. The positions of the training stick are indicated in this schematic drawing. Please click here to view a larger version of this figure.
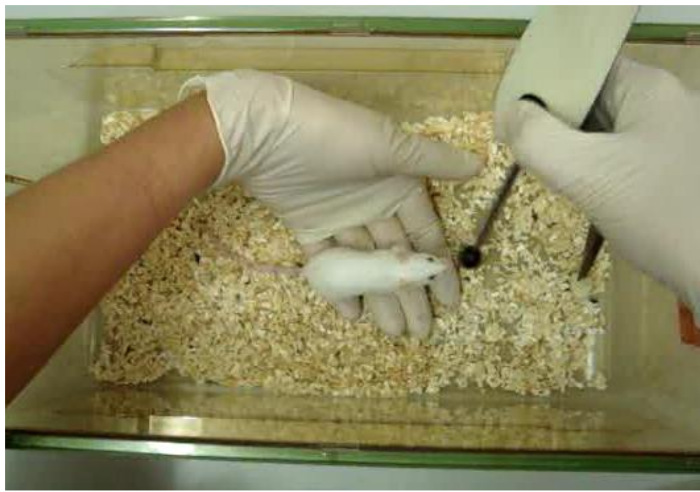
Figure 7. Training Success. A mouse has followed the target stick onto the hand of the trainer. Please click here to view a larger version of this figure.
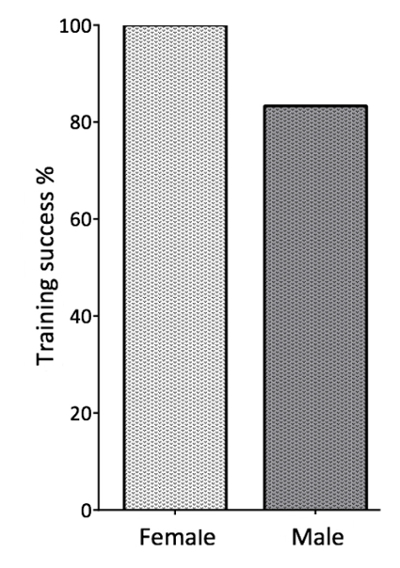
Figure 8. Success of Training Protocol. A cohort of 12 BALB/c inbred strain mice of each sex was trained. By the end of training session week 2: "Following a target stick," all female mice and 83% of the male mice successfully overcame the challenge. Please click here to view a larger version of this figure.
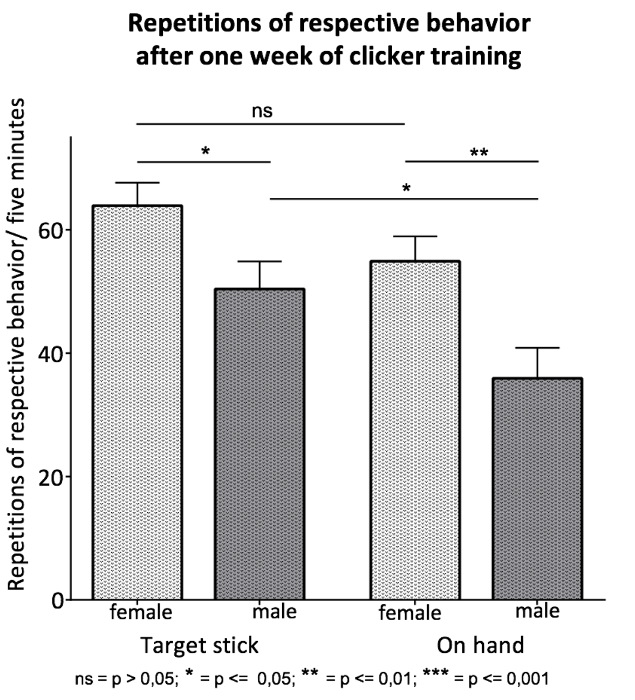
Figure 9. Repetitions of Respective Behavior after One Week of Training. A cohort of 12 BALB/cin bred mice of each sex was trained. On the last day of training session week 2: "Following a target stick" and 3: "Following the target stick to the hand", the repetitions of the respective behavior were counted during a 5-min training session. In both weeks, female mice displayed the strengthened behavior with a significantly higher frequency than male mice ("Target stick":63.92 ± 3.72, p = 0.0300; "On hand": 54.92 ± 4.01, p = 0.0069). The frequency of the strengthened behavior did not significantly vary in female mice from week two to week three. However, male mice showed a significant decrease in repetitions from week two to week three ("Target stick": 50.42 ± 4.48; "On hand": 35.92 ± 5; p = 0.0408). A Mann-Whitney U-test was performed. The results of the data were expressed as the mean ± S.E.M. Please click here to view a larger version of this figure.
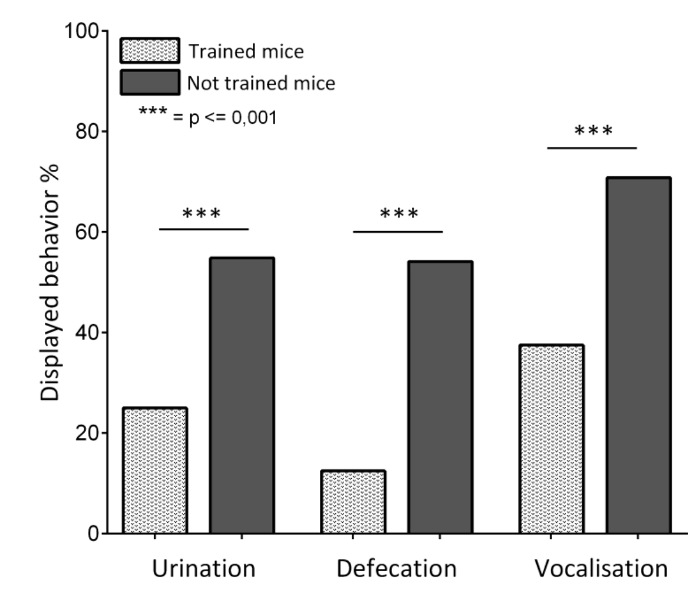
Figure 10. Displayed Behavior Triggered by Scruff Holding. A cohort of 12 BALB/c inbred mice of each sex was trained following the clicker training protocol. An untrained but gently handled cohort of 12 BALB/c inbred mice of each sex served as a control group. The mice were singlehandedly restrained (i.e., grasping the scruff of the neck and the base of the tail with one hand) for 15 s. Spontaneous urination, defecation, and vocalization were recorded. There was a profound difference between the displayed behavior of the trained and the control group. Trained mice displayed all behaviors with a significantly lower frequency than untrained mice. ("Urination": p < 0.001; "Defecation": p < 0.001; "Vocalization": p < 0.001). A Mann-Whitney U-test was performed. The columns are expressed as the percent of all tested mice. Please click here to view a larger version of this figure.
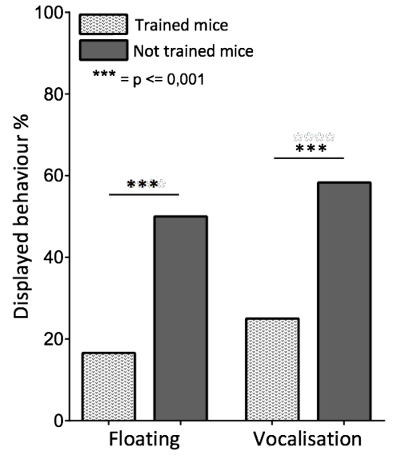
Figure 11. Displayed Behaviors During the Morris Water Maze Test. A cohort of 12 BALB/c inbred mice of each sex was trained following the clicker training protocol. An untrained but gently handled cohort of 12 BALB/c inbred mice of each sex served as a control group. A Morris Water Maze Test was performed with all mice after the third week of training. Floating behavior and vocalization linked to handling were recorded. Floating behavior occurred significantly less in trained mice (p < 0.001). Further, there was a profound difference in the vocalization of the trained and the control group. Trained mice squeaked significantly less when handled than mice of the untrained control group (p < 0.001). A Mann-Whitney U-test was performed. Please click here to view a larger version of this figure.
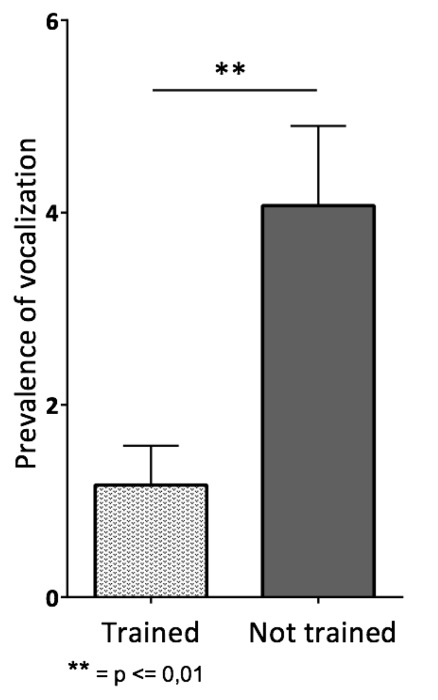
Figure 12. Prevalence of Squeaks per Mouse. A cohort of 12 BALB/c inbred mice of each sex was trained following the clicker training protocol. An untrained but gently handled cohort of 12 BALB/c inbred mice of each sex served as control group. A Morris Water Maze Test was performed with all mice after the third week of training. The vocalization linked to the handling of the mice was recorded. Untrained mice squeaked significantly more often than trained mice. Untrained mice displayed vocalization 1.167 ± 0.7 times and untrained mice 4.071 ± 0.83 times. An unpaired t-test with Welch's correction was performed. The results of the data were expressed as the mean ± S.E.M. Please click here to view a larger version of this figure.

Figure 13. Further Applications. A mouse is following a target stick to cross a bridge from one cage to another. Please click here to view a larger version of this figure.
Discussion
The effective implementation of the clicker training protocol with a cohort of 12 BALB/c inbred mice of each sex proved to be uncomplicated. Previous studies have confirmed the effectiveness of clicker training with several species, and we extended this to mice. As mice are the lowest developed mammals among laboratory animals, their abilities are often underestimated. Therefore, the most surprising aspect of the data is that training success could be achieved with almost all mice.
One critical step within this protocol is the timing of the second reinforcer, which is very important to establishing a connection between the displayed behavior and the reinforcement. As mice are very agitated, it is slightly difficult to mark an exact performance while they are scampering around. The more experienced the trainer is, the faster training success can be achieved. We observed that the mice learned quite quickly, even with unexperienced trainers. Even little mistakes could be compensated for in the course of the clicker training protocol. Common sources of error included reinforcing a wrong behavior and a lack of interest in the food reward after clicking.
An uncontrollable factor is the individual character of each mouse. Although this study was performed with inbred mice, born and raised under the same conditions, they develop a variety of different characters. Due to previous research and our own empirical data, we know that this includes different tastes, different expressions of appetite, and different exploration behavior29,30. The first critical step was to find an appropriate food reward. The mice were offered different kinds of nuts, a sugar solution, marmalade, and different kinds of chocolate. For the mice in this study, white chocolate proved to be the most appropriate reward. The success of this protocol depends on the interest of the mice in the training. If anything else arouses their interest more, the training reaches its limits. The more equipment that was added to the cage to fulfill a training session, the less the mice participated in the training. The elimination of all attention-grabbing items in the surrounding is essential (e.g., leaving even a small amount of bedding material in the cage to prevent the mice from burrowing). Sometimes the mice gain more interest in the training after a habituating to the new environment for several minutes.
Gentle handling protocols have already been determined to benefit the wellbeing of mice when treated amicably9,10,11. The results of the present study indicate that a closer human-animal relationship contributes to mouse tolerance to treatments. Under manipulation, trained animals displayed fewer anxiety-related behaviors (i.e., urination, defecation, and vocalization)31. In the Morris Water Maze Test, the untrained but gently handled mice expressed a significantly higher prevalence of floating behavior. Trained mice displayed less of this depression-related behavior32. It could be argued that the positive results were due to increased well-being in the trained mice.
A limitation of this study is that only one strain of inbred mice was studied. Due to high behavioral differences between different strains of mice, additional inbred strains, as well as outbred strains, should be investigated29. In further studies, supplementary information about mouse wellbeing, such as a whole behavioral battery, physiological data (e.g., corticosterone levels), or the impact of training on brain development, should be gathered.
Further applications of clicker training could assist in improving health and hygiene conditions in animal facilities. By applying the clicker training protocol, we succeeded in teaching mice to follow a target stick and to cross a bridge from one cage to another (Figure 13). If this technique is perfected, it makes manual cage-changing unnecessary. In addition, mice could possibly be trained to voluntarily walk onto measurement equipment (e.g., scale or behavioral areas). This could provide reliable protection against anthropozoonotic disease transmission, as direct contact with the animals will no longer be necessary.
Positive reinforcement training is highly recommended for captive animals, as it contributes to their mental health33. If mice experience a high level of wellbeing, they display few individually different coping strategies. Clicker training, therefore, makes a noteworthy contribution to the 3Rs, as previous work shows that a reduction in the variability of mice phenotypes leads to a reduced number of animals necessary to meet statistical requirements27. This study has demonstrated that clicker training in mice strongly correlates with a reduced fear of human-mice interactions and matches results observed in earlier studies with different species34. Clicker training has further potential, as it could be considered cognitive enrichment. The broad implementation of positive reinforcement training in laboratory animal facilities could make a valuable contribution to the 3R principle, as it refines the keeping and biomedical research of mice.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Translational Animal Research Center of the University Medical Center of the Johannes Gutenberg-University Mainz. The authors are most grateful to Thomas Wacker for his technical support.
Materials
| Lid for open housing | Tecniplast | GM500LID117 | |
| SealSAfe Plus top for open housing | Tecniplast | GM500400SU | |
| Type II long, filter top cages | Tecniplast | GM500PFSPC | |
| Aspen bedding material | Lab & Vet Service GmbH | H0234-300 | Environmental enrichment |
| Red polycarbonate Mouse House | Tecniplast | ACRE011 | Environmental enrichment |
| Tissue papers | Tork, SCA Hygiene Products GmbH | 290179 | Environmental enrichment |
| Food – ssniff M-H Extrudat | ssniff | V1126-000 | ad libitum |
| Target Stick with Clicker | Trixie | 2282 | |
| PVC Tube (Tunnels) | Thyssen Krupp | RTPVCU04003005 | |
| White Chocolate/ white chocolate cream | Company doesn't matter, preferable organic quality | ||
| Forceps | FineScienceTools | e.g. 11150-10 | Or any other tool to fixate chocolate |
| Prism Version 6.0 for Windows | GraphPad Software |
References
- Russell, W. M. S., Burch, R. L. . The Principles of Humane Experimental Technique. , 25-27 (1959).
- European Commission. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union. , (2010).
- Balcombe, J. P., Barnard, N. D., Sandusky, C. Laboratory routines cause animal stress. Contemporary topics in laboratory animal science / American Association for Laboratory Animal Science. 43 (6), 42-51 (2004).
- Meijer, M. K., Sommer, R., Spruijt, B. M., van Zutphen, L. F. M., Baumans, V. Influence of environmental enrichment and handling on the acute stress response in individually housed mice. Laboratory animals. 41 (2), 161-173 (2007).
- Gouveia, K., Hurst, J. L. Reducing mouse anxiety during handling: effect of experience with handling tunnels. PloS one. 8 (6), e66401 (2013).
- Meaney, M. J., Diorio, J., et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Developmental neuroscience. 18 (1-2), 49-72 (1996).
- Gärtner, K., Büttner, D., Döhler, K., Friedel, R., Lindena, J., Trautschold, I. Stress response of rats to handling and experimental procedures. Laboratory animals. 14 (3), 267-274 (1980).
- Izumi, J., Hayashi-kuwabara, Y. U., Yoshinaga, K., Tanaka, Y., Ikeda, Y. Evidence for a Depressive-like State Induced by Repeated Saline Injections in Fischer 344 Rats. Nature. 57 (4), 883-888 (1997).
- Fridgeirsdottir, G. A., Hillered, L., Clausen, F. Escalated handling of young C57BL/6 mice results in altered Morris water maze performance. Upsala journal of medical sciences. 119 (1), 1-9 (2014).
- Heredia, L., Torrente, M., Domingo, J. L., Colomina, M. T. Individual housing and handling procedures modify anxiety levels of Tg2576 mice assessed in the zero maze test. Physiology and Behavior. 107 (2), 187-191 (2012).
- Maurer, B. M., Döring, D., Scheipl, F., Küchenhoff, H., Erhard, M. H. Effects of a gentling programme on the behaviour of laboratory rats towards humans. Applied Animal Behaviour Science. 114 (3-4), 554-571 (2008).
- Skinner, B. F. How to Teach Animals. Scientific American. 185, 26-29 (1951).
- Bailey, R. E., Gillaspy, J. A. Operant psychology goes to the fair: Marian and Keller Breland in the popular press, 1947-1966. The Behavior Analyst. 28 (2), 143-159 (2005).
- McGreevy, P. D., Boakes, R. A. . Carrots and sticks: principles of animal training. , (2007).
- Gillis, T. E., Janes, A. C., Kaufman, M. J. Positive reinforcement training in squirrel monkeys using clicker training. American journal of primatology. 74 (8), 712-720 (2012).
- Schapiro, S. J., Bloomsmith, M. A., Laule, G. E. Positive reinforcement training as a technique to alter nonhuman primate behavior: quantitative assessments of effectiveness. Journal of applied animal welfare science: JAAWS. 6 (3), 175-187 (2003).
- Martin, L., Iceberg, E. Quantifying Social Motivation in Mice Using Operant Conditioning. Journal of visualized experiments : JoVE. (102), e53009 (2015).
- Sclafani, A., Ackroff, K. Operant licking for intragastric sugar infusions: Differential reinforcing actions of glucose, sucrose and fructose in mice. Physiology and Behavior. , 115-124 (2016).
- Bathellier, B., Tee, S. P., Hrovat, C., Rumpel, S. A multiplicative reinforcement learning model capturing learning dynamics and interindividual variability in mice. Proceedings of the National Academy of Sciences of the United States of America. 110 (49), 19950-19955 (2013).
- Clark, F. E. . Can cognitive challenge enhance the psychological well-being of large-brained mammals in zoos? [unpublished doctoral thesis]. , (1999).
- Shettleworth, S. J., et al. . Cognition, evolution, and behavior. , (2010).
- Hagen, K., Broom, D. M. Emotional reactions to learning in cattle. Applied Animal Behaviour Science. 85 (3-4), 203-213 (2004).
- Ernst, K., Puppe, B., Schön, P., Manteuffel, G. A complex automatic feeding system for pigs aimed to induce successful behavioural coping by cognitive adaptation. Applied Animal Behaviour Science. 91, 205-281 (2005).
- Puppe, B., Ernst, K., Schn, P. C., Manteuffel, G. Cognitive enrichment affects behavioural reactivity in domestic pigs. Applied Animal Behaviour Science. 105 (1-3), 75-86 (2007).
- Zebunke, M., Puppe, B., Langbein, J. Effects of cognitive enrichment on behavioural and physiological reactions of pigs. Physiology and Behavior. 118, 70-79 (2013).
- Manteuffel, G., Langbein, J., Puppe, B. Increasing farm animal welfare by positively motivated instrumental behaviour. Applied Animal Behaviour Science. 118 (3-4), 191-198 (2009).
- Bayne, K., Würbel, H. The impact of environmental enrichment on the outcome variability and scientific validity of laboratory animal studies. Science Direct. 33 (1), 273-280 (2014).
- Hurst, J. L., West, R. S. Taming anxiety in laboratory mice. Nature Methods. 7 (10), 825-826 (2010).
- Crawley, J. N. . What’s Wrong With My Mouse?. , (2007).
- Loos, M., Koopmans, B., et al. Within-strain variation in behavior differs consistently between common inbred strains of mice. Mammalian genome : official journal of the International Mammalian Genome Society. 26 (7-8), 348-354 (2015).
- Seibenhener, M. L., Wooten, M. C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. Journal of visualized experiments : JoVE. (96), e1-e6 (2015).
- Can, A., Dao, D. T., Arad, M., Terrillion, C. E., Piantadosi, S. C., Gould, T. D. The Mouse Forced Swim Test. Journal of Visualized Experiments. , 4-8 (2011).
- Clayton, L. A., Tynes, V. V. Keeping the Exotic Pet Mentally Healthy. Veterinary Clinics of North America: Exotic Animal Practice. 18 (2), 187-195 (2015).
- Ward, S. J., Melfi, V. The implications of husbandry training on zoo animal response rates. Applied Animal Behaviour Science. 147 (1-2), 179-185 (2013).

