Failure of Cleaning Verification in Pharmaceutical Industry Due to Uncleanliness of Stainless Steel Surface
Summary
The lack of a well-defined procedure that consistently cleaned coupon surfaces was identified as the major contributor to low and variable recoveries in cleaning verification. This manuscript describes the correct protocol of cleaning stainless steel coupons.
Abstract
The aim of this work is to identify the parameters that affect the recovery of pharmaceutical residues from the surface of stainless steel coupons. A series of factors were assessed, including drug product spike levels, spiking procedure, drug-excipient ratios, analyst-to-analyst variability, intraday variability, and cleaning procedure of the coupons. The lack of a well-defined procedure that consistently cleaned the coupon surface was identified as the major contributor to low and variable recoveries. Assessment of cleaning the surface of the coupons with clean-in-place solutions (CIP) gave high recovery (>90%) and reproducible results (Srel≤4%) regardless of the conditions that were assessed previously. The approach was successfully applied for cleaning verification of small molecules (MW <1,000 Da) as well as large biomolecules (MW up to 50,000 Da).
Introduction
The cleanliness of non-dedicated equipment should be verified before its subsequent release for use in the manufacture of intermediates and active pharmaceutical ingredient (APIs), at product change over to prevent cross-contamination. Cleaning procedures should contain sufficient details to enable operators to clean each type of equipment in a reproducible and effective manner, and these procedures should be validated according to the U.S. Food and Drug Administration (FDA) requirements1. Numerous warning letters due to inadequate cleaning2,3,4, failure to validate the cleaning verification method, and failure to follow cleaning procedures5 have been issued by the FDA. 21 CFR §211.67 outlines the requirements needed for successful cleaning verification.
It is the standard in industry that the validation of the analytical methods for cleaning verification is performed on stainless steel coupons with the same surface/finish as the manufacturing equipment. Stainless steel coupons (e.g., 50 cm2) are used to represent equipment surfaces for cleaning verification experiments in the laboratory. During the development and validation of these analytical methods, the sample of interest (i.e., the residues that should be recovered from the surface of the equipment) is spiked at the target residue onto the stainless coupon determined by the maximum allowable carry-over (MACO) limit. This level is determined based on acceptable exposure limit which is defined as the limit at which a patient can get exposed with no adverse health effects (no-observed-adverse-effect-level, NOAEL).
The analyst or manufacturing operator conducting the swabbing must follow a structured procedure to ensure that recoveries are reproducible regardless of who performs the swabbing. The procedure should explicitly detail the swab type, number of swabs used, the diluent, the amount of solvent used, the exact sweeping pattern, the number of strokes applied to the sampling surface, the amount of time spent swabbing/extracting the samples, the method of detection (ultra-violet, fluorescence, mass spectrometry, total organic carbon, etc.), the extraction technique of the material from the swab head, etc.
Besides all the above-mentioned factors that affect sample recovery, the surface of the coupon, and thus, the surface of the equipment also play a role. The surface of the coupon may be modified due to the deposition of a thin film of material on the surface or due to change in the oxidation state of one or more of the elements in stainless steel (e.g., Fe, Cr, and Ni)6,7,8. The regeneration of the surface of the stainless steel coupons back to its original state is vital for the success of the quantitative swapping process. Studies, in which the stainless steel coupons were not properly cleaned, showed variability in recovery including analyst to another, different drugs, or various spike levels9,10,11. The standard deviation in recovery of ten replicates on one coupon can be up to 14% and 26% on five coupons9. It is important to note that the relative standard deviation (Srel) values increased with increasing number of replicates or with increasing the number of coupons used (i.e., five coupons instead of spiking five times on the same coupon)11. In such cases, the variability cannot be interpreted as random fluctuation. Nonetheless, their results can be explained by our finding that the cleanliness of the surface of the coupon will affect the recovery. The results described in this paper illustrate a significant increase in recovery results and decrease in variability after properly cleaning the surface of the stainless steel coupons.
Clean-in-place (CIP) is an automated way of cleaning the surface of equipment that involves a minimal or no disassembling of the equipment. During the CIP cleaning process, a defined procedure of consecutive wash with a base followed by an acid is executed to remove organic and inorganic residues. Surfactants, chelating compounds, or complexing agents are usually added to the CIP solutions to enhance the efficiency of cleaning any product from the surface of the equipment. The efficiency of cleaning depends on several parameters including the choice and concentration of the CIP solutions (i.e., type and composition of base, acid, and surfactant), the cleaning time, temperature (typically 60 – 80 °C), contamination type, and the presence of hard to clean parts12. Based on the type of drug product, CIP solutions 100 and 200 have been chosen to use for cleaning the stainless steel coupons used for cleaning verification, since it simulates the CIP process used for cleaning the manufacturing equipment.
This study reports the influence of different factors affecting the recovery of pharmaceutical residues from the surface of stainless steel coupons and recommends the best practices for analytical cleaning method development for small molecules, therapeutic proteins, and antibodies. The lack of a well-defined procedure that consistently cleaned coupon surface was identified as the major contributor to low and variable recoveries. High and reproducible recovery was obtained when the surface of the coupon was cleaned properly13.
Protocol
1. Sample Solution
- Calculate the cleaning limit (CL) for a drug based on the maximum allowable carry-over (MACO) according to the therapeutic dose criteria.
NOTE: Here, the cleaning limit (CL) for drug A has been calculated to be 2.4 µg per 50 cm2 based on the maximum allowable carry-over (MACO) according to the therapeutic dose criteria. The cleaning verification is executed at the 50%, 100%, and 150% cleaning limit. The cleaning verification has been assessed for two formulations (2.5% and 60% w/w drug load).
2. Cleaning Procedure for Coupons
- Initial approach
- Clean the coupons by rinsing and wiping the surface for 10 – 15 s twice with water and twice with methanol to eliminate any residual deposit. Perform this process in the hood as methanol is toxic and highly volatile.
- Advanced approach
- Using clean-in-place solutions
- Set the sonicator at room temperature. The sonicator has no power setting adjustment, therefore the time is set to give adequate cleaning results.
- Immerse the coupons in high performance liquid chromatography (HPLC) grade water and sonicate for 2 min.
- Immerse the coupons in 0.1% alkaline detergent solution in HPLC grade water and sonicate for 2 min.
- Immerse the coupons in HPLC water and sonicate for 2 min.
- Immerse the coupons in 0.1% acid detergent solution in HPLC water and sonicate for 2 min.
- Immerse the coupons in HPLC water and sonicate for 2 min.
- Using acid-base-peroxide
NOTE: This is an alternative method included using acid, base, and peroxide rather than alkaline and acid detergents.- Set the sonicator at room temperature. The sonicator has no power setting adjustment, therefore the time is set to give adequate cleaning results.
- Immerse the coupons in water and sonicate for 2 min.
- Immerse the coupons in 0.1 M sodium hydroxide and sonicate for 2 min.
- Immerse the coupons in water and sonicate for 2 min.
- Immerse the coupons in 0.1 M hydrochloric acid and sonicate for 2 min.
- Immerse the coupons in water and sonicate for 2 min.
- Immerse the coupons in 0.1 mg/mL NaNo2 and sonicate for 2 min.
- Immerse the coupons in water and sonicate for 2 min.
- Using clean-in-place solutions
3. Swabbing Procedure
- Mount the stainless steel coupons on the bottom of a 250 mL (or 500 mL) plastic beaker using a double sided tape.
- Hold onto the beaker to make spiking and swabbing processes easy. This also minimizes some undesirable accidents such as overshooting the corner when swabbing.
- Infuse a defined volume at a specific concentration and formulation (e.g., 200 µL of 12 µg/mL at 2.5 % w/w drug A load) in a whirly pattern on the surface of the coupon using a positive displacement pipette.
- Wait until the surface of the coupon is dry (~ 3 – 10 min depending on the volatility of the sample).
- Dip a dry swab in a vial with 2 mL of diluent (methanol:formic acid 100:0.2 volume/volume).
- Remove excess solvent by pressing the swab against the inside of the vial.
- Firmly wipe the surface of the coupon with even, overlapping side-to-side strokes until the total 50 cm2 test area is wiped with one side of a wet swab.
- Repeat the wiping process (step 3.7) using the same side of the swab.
- Swab the four edges of coupon twice.
- Flip the swab to the other side and rotate the coupon 90°, clockwise or counterclockwise; thus, rotating the direction of swabbing by 90°.
- Repeat the swabbing motion as detailed in steps 3.7 – 3.9. After the sampling of the surface, cut the swab head using scissors into the solvent vial.
- Repeat the swabbing process with a second swab while rotating the coupon 90° toward the same direction chosen before.
- Sonicate the vial containing two swab heads for 5 min, then vortex for 10 s.
- Transfer the solution into an HPLC vial and label it as working solution.
4. Calculation of Recovery
- Sample recovery
- Prepare the control solutions by mixing 200 µL each of the coupon spiking solutions with 1,800 µL of diluent.
- Run the chromatography system according to the conditions listed in Table 1.
- Calculate the recovery of the swab working solution based on the relative area under peak of the working solutions (AW) and the control solution (AC).
- Repeat the process on three coupons and calculate the average recovery along with relative standard deviation (Srel).
NOTE: All the analytical methods used here were validated according to the ICH Q2(R1) guidelines. The chromatography conditions are listed in the material section.
Representative Results
Representative results from the initial attempts for cleaning verification for drug A are summarized in Table 2. Before the coupons were cleaned according to the detailed procedure in the experimental section, inconsistent results were obtained at different spike levels, for various ratios of API/excipient, different analysts, and even for the same analyst at different days. The observed variability in the recoveries needed to be addressed, as some of the results failed the validation requirements (60% < recovery < 150%), such as the recovery for the 60% drug load at all cleaning limits.
The first type of variability observed in Table 2 is the variability in precision as can be seen from the high Srel associated with the majority of the recovery results (numbers listed in parentheses). Besides to the expected analyst-to-analyst variability (data shown in reference 13), day-to-day variability is also observed for one analyst with all other conditions not changed, as seen in the first two experiments in Table 2 (at 2.5% drug load). Inconsistent recoveries were observed at the different spike levels of 50%, 100%, and 150% of the cleaning limit (Srel up to 16% for the 60% drug load at 150% cleaning limit), regardless of the drug load or the analyst doing the experiment. In addition, there was variability even at different API/excipient ratios, at 2.5% and 60% drug load (Table 2) and at 50% drug load reported in reference 13). The low formulation gave the highest recovery on average, suggesting that the excipient was enhancing the recovery of the drug from the coupon. Quite possibly 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC), a surfactant, shielded the organic drug compound from metal chelation interaction and enhanced the removal of the drug from the coupon surface when the drug/excipient ratio was lower.
Based on the types of variability discussed above, the initial approach to improve recovery was to redevelop the extraction method and the experimental conditions to obtain consistent and high recoveries. Adjusted parameters included: swabbing technique, the diluent (different solvents, various organic/aqueous ratios, different acids and acid concentration), the spiking solvent, the pH of the spike and the diluent, the spiking technique, and the extraction technique of drug from swab. The average recoveries on four different coupons along with relative standard deviation are shown in Figure 1 for some experiments. The main conclusion was that none of the aforementioned changes eliminated the previously observed variability in Table 2. Regardless of the experimental factor that was changed, the variability in recovery (Srel) from one coupon to another was evident and in some cases it was >20%. Within experimental error, almost all of these experiments were not considered statistically different. The difference between the individual recovery at each coupon surface and the average recovery (ΔRecovery) are shown in Figure 2. It is clear that the average recovery is different from one coupon surface to another. Therefore, the coupon surface is expected to be a major contributor to the observed variability.
There was a high probability that the previously observed variability in recovery was due to coupon-to-coupon variability. The cleaning verification experiments for 60% drug load were repeated six times, with each formulation spiked onto four coupons of identical material and surface finish, all from the same vendor. It was clear from the results shown in Figure 3 that the recovery for the 60% formulation was not very reproducible from one trial to another, with a general trend towards lower recoveries as the experiments progressed. Moreover, some differences were observed between coupons at this formulation (Figure 2). The observed variability suggested that the surface of the various coupons was not identical and interacted differently with the matrix.
The first approach to minimize the difference among the coupons was to thoroughly clean the surfaces of the coupons. Coupons used for obtaining recovery in Figure 3 were cleaned according to the procedure presented in the experimental section. The recovery results after cleaning the coupons are presented in Figure 3. It is clear that the recovery is virtually reproducible from one trial to another and that the difference in recovery between coupons is minimized.
Table 2 shows a comparison of the recovery results before and after cleaning the coupons under the same experimental conditions. The following conclusions can be drawn: 1) All the recoveries were high (90 – 100%); 2) The Srel values at each spiking level were acceptable and much smaller than the previously reported results on uncleaned coupons, 3) The variability in the recovery from one spike level to another was minimized, 4) The difference in formulation did not affect the recovery.
Coupons cleaned with CIP solutions were then used for cleaning verification of compounds B (another small molecule) C and D (large molecules, i.e., biologics) at different formulations and spike levels. The same conclusions drawn from experiments for drug A were applicable for drug B, C, and D (detailed results shown in reference 13). High recoveries were obtained across molecular size and physicochemical properties by applying a systematic cleaning approach for the coupons.
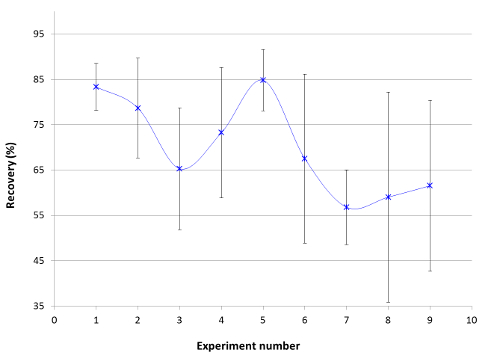
Figure 1. Average Recovery Obtained from Four Coupons. Error bars represent relative standard deviation from four trials on four coupons. Experiment number: 1) normal spike, 2) 10% formic acid, 3) no formic acid, 4) no water in spike solution, 5) high placebo percentage (97%), 6) no placebo, 7) squeezing swab with spatula, 8) adding a centrifuging step, and 9) 0.1% HCL. Please click here to view a larger version of this figure.
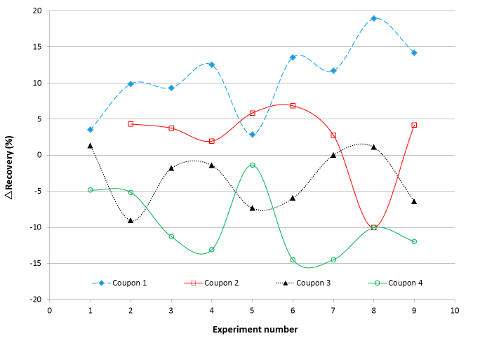
Figure 2. Difference in Recovery (ΔRecovery) Obtained from Four Coupons. ΔRecovery is the difference between the recovery on the coupon and the average recovery of the four coupons. Experiment number: 1) normal spike, 2) 10% formic acid, 3) no formic acid, 4) no water in spike solution, 5) high placebo percentage (97%), 6) no placebo, 7) squeezing swab with spatula, 8) adding a centrifuging step, and 9) 0.1% HCL. Please click here to view a larger version of this figure.
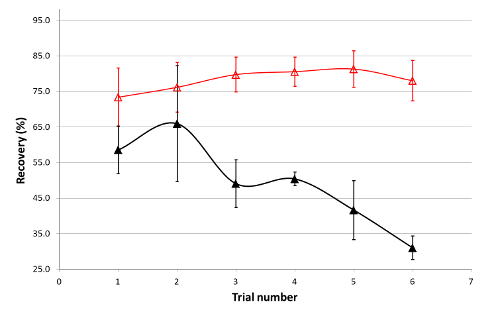
Figure 3. Variability in Recovery of Drug A at 2.5 % Drug/Excipient Ratio Before and After Cleaning. Solid triangles correspond to recovery values before cleaning the coupons, while open symbols correspond to the recovery values after cleaning the coupons. Error bars represent relative standard deviation from four trials on four coupons. Please click here to view a larger version of this figure.
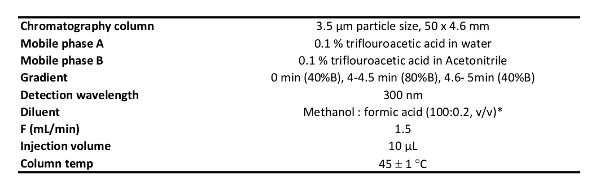
Table 1: Chromatography Conditions.
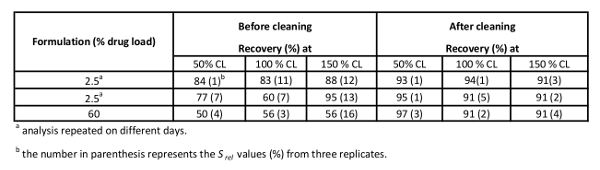
Table 2. Spiked Recoveries for Different Drug Load Before and After Cleaning the Coupons According the Procedure in the Experimental Section.
Discussion
The major contributor to low and variable recoveries of API residues from stainless steel coupons was traced to the lack of a well-defined procedure for cleaning of the coupon surfaces. Cleaning the surface of the coupons resulted in consistent, accurate spiked recovery and reproducible results. With the demonstration of high recoveries from stainless steel coupons, the actual cleaning verification results obtained from the manufacturing equipment using validated method(s) should be accurate and precise, reflective of the residue level on the equipment with minimum risk of false negatives for product to product carryover that could jeopardize patient safety.
The initial approach followed by the analyst to troubleshoot the low and inconsistent recovery was by modifying the experimental conditions such as: percent of organic in the diluent, the spiking process, the type and strength of acid used in the spiking solution, etc. This approach did not solve the low and inconsistent recovery issue. Nonetheless, this aforementioned problem was completely solved when the stainless steel was adequately cleaned using clean-in-place solution. This achievement results in vastly increasing the chances of the successful pass of cleaning verification of the manufacturing equipment. It is important to note that the CIP solutions used here are limited to the pharmaceutical industry and thus suitable CIP solutions should be selected for other types of industries (processed food, dairy, cosmetics, etc.). The choice of CIP solutions and the cleaning process are critical steps for the success of this process. The protocol presented here will help analysts in the pharmaceutical industry as well as other industries to better design and execute successful cleaning verification. The work will also help minimizing batch-to-batch and/or product-to-product cross-contamination that may adversely affect the human health.
Disclosures
The authors have nothing to disclose.
Acknowledgements
No funding agencies supported this work.
Materials
| stainless steel coupons | GlobePharma (New Brunswick, NJ ). | SS316-20RA-50cm2 | |
| Clean in place solutions (CIP100 and CIP200) | were obtained from Steris Corporation (Mentor, OH) | 1D10BG | Alkaline detergent and acid detergent, respectively |
| Positive displacement pipettes | Gilson (Middleton, WI). | ||
| HPLC grade water | Millipore Milli-Q Advantage Water Purification System (Darmstadt, Germany) or from Honeywell Burdick & Jackson (Muskegon, Michigan) | 7732-18-5 | |
| HPLC grade Methanol | EMD | MX0475-1 | |
| glacial acetic acid | EMD | MAX0073P5 | |
| HPLC grade Acetonitrile | J.T. Baker (Avantor Performance Materials, Center Valley, PA) | 75-05-8 | |
| Trifluoroacetic acid | J.T. Baker (Avantor Performance Materials, Center Valley, PA) | 75-05-8 | |
| Chromatography column Zorbax Eclipse | XDB-C18, 4.6 x 100 mm, 3.5 µm HPLC column | UNSPSC – 41115709 | |
| Vanquish UHPLC system | Thermo Fisher Scientific, Germering, Germany | ||
| Branson B8510 Ultrasonic cleaner | Branson Ultrasonics (Danbury, CT, USA) | model (8510-D7H) |
References
- . . Validation of cleaning processes (7/93). Guide to inspections validation of cleaning processes. , (2004).
- . . Warning Letter: 320-12-08, Gulf Pharmaceuticals. , (2012).
- . . Warning Letter: 320-12-058, Novartis Internationsl AG. , (2012).
- . . Warning Letter: 05-10, Teva Parenterals Medicines, Inc. , (2009).
- . . Warning Letter: 320-14-09, Tianjin Zhongan Pharmaceutical Co., Ltd. , (2014).
- Mantel, M., Wightman, J. Influence of the surface chemistry on the wettability of stainless steel. Surf Interface Anal. 21, 595-605 (1994).
- Odaka, K., Ueda, S. Dependence of outgassing rate on surface oxide layer thickness in type 304 stainless steel before and after surface oxidation in air. Vacuum. 47, 689-692 (1996).
- Kerber, S. J., Tverberg, J. Stainless Steel Surface Analysis. Adv. Mater. Processes. , 33-36 (2000).
- Kusumaningrum, H. D., et al. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol. 85, 227-236 (2003).
- Liu, L., Pack, B. W. Cleaning verification assays for highly potent compounds by high performance liquid chromatography mass spectrometry: Strategy, validation, and long-term performance. J Pharmaceut. Biomed. 43, 1206-1212 (2007).
- Lambropoulos, J., Spanos, G. A., Lazaridis, N. V. Development and validation of an HPLC assay for fentanyl, alfentanil, and sufentanil in swab samples. J Pharmaceut. Biomed. 23, 421-428 (2000).
- Chisti, Y., Moo-Young, M. Clean-in-place systems for industrial bioreactors: Design, validation and operation. J. Ind. Microbiol. 13, 201-207 (1994).
- Haidar Ahmad, I. A., et al. Cleaning verification: Exploring the effect of the cleanliness of stainless steel surface on sample recovery. J Pharmaceut. Biomed. 134, 108-115 (2017).

