Transcranial Electrical Brain Stimulation in Alert Rodents
Summary
This protocol describes a surgical set-up for a permanent epicranial electrode socket and an implanted chest electrode in rodents. By placing a second electrode into the socket, different types of transcranial electrical brain stimulation can be delivered to the motor system in alert animals through the intact skull.
Abstract
Transcranial electrical brain stimulation can modulate cortical excitability and plasticity in humans and rodents. The most common form of stimulation in humans is transcranial direct current stimulation (tDCS). Less frequently, transcranial alternating current stimulation (tACS) or transcranial random noise stimulation (tRNS), a specific form of tACS using an electrical current applied randomly within a pre-defined frequency range, is used. The increase of noninvasive electrical brain stimulation research in humans, both for experimental and clinical purposes, has yielded an increased need for basic, mechanistic, safety studies in animals. This article describes a model for transcranial electrical brain stimulation (tES) through the intact skull targeting the motor system in alert rodents. The protocol provides step-by-step instructions for the surgical set-up of a permanent epicranial electrode socket combined with an implanted counter electrode on the chest. By placing a stimulation electrode into the epicranial socket, different electrical stimulation types, comparable to tDCS, tACS, and tRNS in humans, can be delivered. Moreover, the practical steps for tES in alert rodents are introduced. The applied current density, stimulation duration, and stimulation type may be chosen depending on the experimental needs. The caveats, advantages, and disadvantages of this set-up are discussed, as well as safety and tolerability aspects.
Introduction
The transcranial administration of electrical currents to the brain (tES) has been used for decades to study brain function and to modify behavior. More recently, applying direct currents, or less frequently alternating currents (tACS and tRNS), noninvasively through the intact skull by use of two or more electrodes (anode(s) and cathode(s)) has gained scientific and clinical interest. In particular, tDCS has been used in more than 33,200 sessions in healthy subjects and patients with neuropsychiatric diseases and has emerged as a safe and easy, cost-effective bedside application, with possible therapeutic potential as well as long-lasting behavioral effects1. This clearly yielded the increased need and scientific interest in mechanistic studies, including safety aspects. This article focuses on the most commonly used form of stimulation, tDCS.
Across species, tDCS modulates cortical excitability and synaptic plasticity. Excitability changes have been reported as polarity-dependent alteration of spontaneous neuronal firing rate in rats and cats2,3,4, or as changes in motor evoked potential (MEP) amplitudes in humans and mice (both increased after anodal and decreased after cathodal tDCS: human5,6; mouse7). Anodal DCS increased synaptic efficacy of motor cortical or hippocampal synapses in vitro for several hours after stimulation or long term potentiation (LTP), when co-applied with a specific weak synaptic input or when given before a plasticity inducing stimulation8,9,10,11,12. In accordance, the benefits of stimulation on motor or cognitive training success are often revealed only if tDCS is co-applied with training8,13,14,15. While these previous findings are mainly attributed to functions of neurons, it should be noted that non-neuronal cells (glia) may also contribute to functional effects of tDCS. For instance, astrocytic intracellular calcium levels increased during anodal tDCS in alert mice16. Similarly, anodal tDCS at current densities below the threshold for neurodegeneration induced a dose dependent activation of microglia17. However, the modulation of neuron-glia interaction by tDCS needs further specific investigation.
Taken together, animal research clearly advanced our understanding of the modulatory effect of tDCS on excitability and plasticity. However, there is an "inverse translational gap" observable in the exponential increase in publications of human tDCS studies in contrast to the slow and minor increase in investigations of the underlying mechanisms of tES in in vitro and in vivo animal models. Additionally, rodent tES models are performed with high variability across research laboratories (ranging from transdermal to epicranial stimulation), and reported stimulation procedures are often not fully transparent hindering the comparability and replicability of basic research data as well as interpretation of results.
Here, we describe in detail the surgical implementation of a transcranial brain stimulation set-up targeting the primary motor cortex, which allows translation to the human tDCS condition while minimizing variability, and allows repeated stimulation without hindering behavior. A step-by-step protocol for subsequent tES in alert rats is provided. Methodological and conceptual aspects of safe application of tES in alert rodents are discussed.
Protocol
For research involving animals, the relevant (country-specific) approvals must be obtained before starting experiments. All animal experiments reported here are performed according to the EU directive 2010/63/EU, the updated German animal protection law ("Tierschutzgesetz") of July 2013, and the updated German animal research regulations of August 2013. Animal protocols have been approved by the local authorities "Commission for Animal Experimentation of the Regional Council of Freiburg" and "Commission for Animal Experimentation of the University Medical Center Freiburg".
1. Preparation of Instrumentation and Material for Surgery
- Make sure that the items listed in Figure 1 are available and already placed for surgery.
- Prepare a thin rectangular platinum plate (e.g., 10 mm x 6 mm x 0.15 mm), which will serve as the counter electrode placed subcutaneously on the chest, and punch two small holes in two opposite corners of the plate.
- Solder an insulated cable with a length of ~10 cm using a lead-free tin-solder to one of the corners (without a hole) of the platinum plate.
- Apply a small drop of histo-acrylic glue on the soldering joint for isolation.
2. Preparation of the Rodent for Surgery
- Assign a study number to the rodent and note this on the prepared surgery card.
- Weigh the rodent and note the weight on the surgery card. Calculate the dose of injection anesthetics (e.g., ketamine 80 mg/kg bodyweight plus xylazine 12 mg/kg bodyweight for rats).
- Induce anesthesia by intraperitoneal (i.p.) injection of the calculated amount of anesthetics.
NOTE: When using inhalation anesthesia instead (e.g., isoflurane), place the rodent in an induction chamber with continuous flow of ~4% in 1-2 L/min oxygen. - Check depth of anesthesia by the toe pinch reflex starting 5 min post injection. If the toe pinch reflex is still present, reach prolongation and deepening of anesthesia by injection of 30% of the initial dose.
- If at any time point in the experiment the toe pinch reflex returns, 30% of the initial dose of anesthesia should be injected.
- When using inhalation anesthesia, look for loss of the postural reflex of the rodent in the induction chamber and check the depth of anesthesia by the lack of a toe pinch reflex. If reflexes are still present, extend the duration in the anesthesia chamber. Throughout the whole experiment, adapt the percentage of isoflurane to the depth of anesthesia until reaching a maintenance concentration of ~1-1.5% isoflurane.
- When the frequency of breathing decreases and gasping occurs, lower the percentage; when the rodent regains the toe pinch reflex or shows spontaneous movement, increase the percentage of inhalation anesthetic.
- As soon as reflexes are absent, place the rodent on the lab bench or hold it in hand.
NOTE: When using inhalation anesthesia, provide continued reduced isoflurane flow (now between 2-3%) by using a nozzle connected to the nebulizer. - Remove the hair on the rat's head by shaving the area from ear to ear and from between the rostral eye level to just behind the ears with a clipper. Then remove the hair on the chest by shaving the area between the forelimbs from the xiphoid up to the clavicles.
NOTE: Keeping the skin under tension eases the shaving. - Cover the eyes of the rat with a drop of eye ointment to protect the cornea.
- Mark the rat's ear according to the assigned study number.
NOTE: Depending on the length of the study, a tail mark might be sufficient, otherwise standardized earmarking is preferable.
3. Surgical Procedure: Chest Electrode Implantation
NOTE: This step can be skipped when the counter electrode is placed externally on the shaved chest with a vest.
- Place the rodent prone (on the chest) on the operation table.
NOTE: In case of inhalation anesthesia, keep the rat's snout placed in the anesthesia nozzle, further reducing the isoflurane concentration to 1.5-2%. - Disinfect the shaved scalp with a disinfectant spray or with a swab soaked in antiseptic agent (e.g., ethanol 70%) and let air-dry. Repeat two times.
- Cut the skin with a scalpel in one line from the rostral eye level to the mid ear level.
NOTE: This allows for tunneling of the connecting cable from the implanted chest electrode toward the top of the head and is also the desired cut for the DCS electrode socket placement. - Turn the rat to supine position, so that the chest is exposed.
- Disinfect the skin of the chest as described in step 3.2.
- Elevate the lateral skin of the right chest with a tissue forceps and cut a buttonhole with small scissors of about 0.5 cm medial from the right axilla. Then make a straight sagittal cut in cranial orientation with the scissors.
- Form a subcutaneous pouch by atraumatically disconnecting the skin from the left major pectoral muscle. Do so by repeatedly opening the small scissors (or by a saline soaked cotton swab).
- Turn the animal on its right side to tunnel the cable path from the left occipital corner of the opened head skin along the neck to exit into the pectoral pouch by penetrating the superficial fascia using homeostatic forceps.
- Carefully open the homeostatic forceps to grab the end of the electrode cable attached to the platinum electrode without allowing sharp wires to stray. Pull the cable through the tunnel until the electrode enters the pouch, oriented with the soldering point towards the rodents left hindlimb. Turn the rodent back to the prone position.
- Fix the platinum plate with a sterile synthetic braided non-absorbable suture to the pectoral fascia at the two opposing corner holes (4-5 knots are recommended for stability).
- Similarly attach the cable to the fascia by a loose knot, forming a slight loop before the entrance of the tissue tunnel.
- Close the skin with 3-4 cutaneous sutures depending on the size of the cut (the same suture material can be used as for the electrode and cable).
4. Surgical Procedure: Placement of the Epicranial tES Socket
- Place the animal in a stereotactic frame.
NOTE: If using inhalation anesthesia, lower the concentration of the anesthetic to a maintenance isoflurane flow of ~1.5-1%, adjusted to the toe pinch reflex and breathing pattern. - Disinfect the shaved scalp as described in step 3.2.
- Cut the skin with a scalpel in one line from the rostral eye level to the mid ear level.
NOTE: If the chest electrode placement was performed, steps 4.2 and 4.3 have already been performed. - Scrape off the periosteum (connective tissue on the skull) to the sides with the scalpel and thoroughly wipe off with cotton swaps. Fixate the connective tissue at the 4 corners of the cut with bulldog clamps and let them hang laterally to keep the surgery field open.
- Apply 0.9% saline to clean the bone surface and tissue with cotton swabs. Then clean the bone surface with 3% H2O2. Avoid contact with the tissue. Hereby the bone is cleaned more thoroughly and minor bleeding from the bone will be stopped. Also, residuals of the periosteum become visible. Remove these residuals with a cotton swab applying moderate pressure.
NOTE: Removal of the periosteum residuals will increase adhesion and durability of the tES socket glued onto the bone.- In case of unstoppable bleeding, use a bone drill and touch it for 1-3 s with slight pressure on the bone. This mechanical procedure will in most cases stop the bleeding without significant heating. Never use electrocautery on the bone; even brief application will result in brain tissue damage (electrocautery should solely be used for wound tissue bleeding).
- As fixation screws will improve set-up adherence, choose a drill bit fitting the screw size. Place two burr holes on two different bone plates by pre-drilling with a hand drill and then by slight vertical pressure application with the bone drill. Avoid close proximity to the desired position of the tES socket, as it might hinder screwing in the electrode (e.g., for left primary motor cortical tES, choose right frontal and posterior parietal screw position).
- In case of an implanted counter electrode, burr a third hole located in the right posterior parietal bone for future fixation of the tunneled cable.
- Place the plastic screws in the burr holes and screw until the first friction is felt. Then perform three additional 180 ° screw turns. Check with forceps for stability of the screw and add one more turn if not tight enough.
NOTE: For adult rats this will ensure epidural placement of the screws without damaging the dura or brain (depending on the screw thread design, the turn number might vary) . The use of stainless steel screws should also be feasible, since even at DCS current densities above the neurodegeneration threshold, screw placement did not perturb lesion location or extent below the screws. - Turn on the soldering iron and pre-heat for approximately 5 min. Wind the cable exiting the tissue tunnel occipitally around the right parietal screw and then cut it, leaving approximately 1 cm cable behind the winding. Carefully strip the insulation at the end of the cable with a scalpel.
- Fix the winded cable to the screw and bone with cyanoacrylic glue.
- Apply a small amount of the lead-free tin-solder to the connector and to the bare wires of the counter electrode cable and connect both by briefly pressing both pre-soldered parts together while touching the soldering tip until the tin-solder melts (about 2-3 s). Remove the soldering tip immediately to avoid excessive metal heating of the cable with subsequent tissue damage.
- Pick up the custom made tES electrode socket (Figure 1B, in red) with bent, serrated tip forceps and apply a thin layer of cyanoacrylic glue to the bottom rim of the socket. For placement above the motor cortex and using a 4 mm diameter socket, place the mid socket point at 2 mm anterior and 2 mm lateral from the bregma. For this position, the inner medial border of the socket should end directly at the sagittal suture and the caudal border should end at the height of bregma. Press the socket briefly onto the bone (most cyanoacrylic glues harden by pressure).
NOTE: Placing a light source directly above the socket can ease placing the socket. - Ensure that the bone within the area of the socket is free of glue (by checking with light because the glue is reflective). In the case of glue spill, remove the socket, scrape the glue with the scalpel, and repeat step 4.12.
- After the socket is in place and the future stimulation area is free of glue, first seal the lateral border of the socket to the neighboring tissue with a small drop of cyanoacrylic glue to avoid a fluid bridge that could lead to shunting of current at this location. Do not apply too much glue as it may flow into the stimulation area (if this occurs, return to step 4.12).
NOTE: Keeping the stimulation area free of glue is crucial as a reduction of the stimulation area might dramatically increase current density (A/m²). - Cover all screws with cyanoacrylic glue.
- Mix the two-component dental acrylic cement in a small silicon tube or glass. As soon as it becomes viscous, apply it with a dental spatula to seal the remaining borders of the socket to the bone. Avoid any flow of dental acrylic cement into the stimulation area.
- Finally cover the whole skull, screws, counter electrode cable and the socket up to ⅓ of the socket with dental acrylic cement. Ensure that the cement has the correct viscosity: if too fluid, it will flow into the surrounding tissue; if too hard it is difficult to distribute it evenly.
- When all the bone is covered and the cement is hardened, remove the bulldog clamps; the skin should just touch the built-up cement so that suturing is not needed. (If the initial cut was chosen too long and the connective tissue or muscle is visible, apply a suture as described in step 3.12).
- Apply one layer of iodine with a cotton swab around the border of the cut skin and subcutaneously inject carprofen (5 mg/kg body weight dissolved in 5-7.5 mL of 0.9% saline for pain treatment and fluid replacement).
NOTE: If using inhalation anesthesia, turn it off now. - Place the rodent in a warming box for recovery from anesthesia until the rodent is awake and postural stability is restored.
NOTE: Check the animal's weight development, wound state, and general well-being criteria daily according to the institution's recommendation.
5. Transcranial Electrical Stimulation Procedure
NOTE: As anesthesia affects tES effects, performing the stimulation in alert rodents whenever possible is recommended. Allow the rodent to recover for at least 5 days (healing of the head and chest wound) before starting experiments. Experiments can be performed at earlier time points after surgery when using an external counter electrode fixed with a vest, as the chest wound is most irritable; but animals need to be habituated to the electrode vest for several days and interference with behavioral tasks might occur.
- Fill the tES electrode socket half with 0.9% saline and remove air bubbles.
- Before cathodal tDCS sessions, always check chlorination, and if needed (such as a shiny silver surface), re-chlorinate the Ag/AgCl electrode. Before anodal tDCS sessions, remove possible excess AgCl deposits from previous stimulations with sandpaper to allow for good conductivity during stimulation. Screw in the tES electrode screw cap (Figure 1B, grey piece).
CAUTION: Failure to re-chlorinate the electrode between cathodal tDCS sessions will lead to exhaustion of chlorination during stimulation and to toxic build-up by electrochemical reaction. This will induce tissue damage. Re-chlorination is not needed within a single session if stimulation duration is shorter than 20 min. - Connect the cables to the two connectors on the head (for anodal stimulation, the anodal cable is connected to the connector on the screw cap, for cathodal stimulation, it is opposite).
NOTE: When using an externally placed counter electrode, cover the counter electrode with conductive gel and place on the rodent's chest. This is easiest if the electrode is pre-fixed in a small rodent vest, which the rodent can wear during stimulation. - Place the rodent into the experimental cage, with the cables connected to a swivel above the cage that allows for free movement.
- Turn on the stimulator and adjust the stimulation parameters (stimulation intensity, duration, ramp up and down time).
- When not using a commercially available stimulation device with a safety shut down and disconnection alarm, include a meter in the circuit to check the constant current flow.
NOTE: With this set-up, stimulation can be applied during performance or training of behavioral tasks. - Check for signs of stress or discomfort of the rodent during stimulation.
- After the end of the stimulation, disconnect the cables, unscrew the electrode cap on the head, and clean and dry the socket with a cotton swab. Return the rodent to the home environment or proceed with a behavioral procedure if desired.
Representative Results
The described implementation of a set-up for reliable repeated tES in alert rodents can be easily integrated into mechanistic experiments, dose-response studies, or experiments including behavioral tasks. To date, the comparability of data from animal studies using (noninvasive) tES is hindered by the variability of the tES stimulation set-ups between laboratories and by differences in stimulation parameters (e.g., various current densities applied at exorbitant high levels compared to the human application). Hence, the informative value of animal research in the field of tES is limited. This article presents a tES set-up which is easy to standardize across laboratories by implementing the placement of the "active" electrode on the bone above the targeted cortex (here, above the primary motor cortex (M1)) with saline as the preferable conductive medium and the counter electrode placed on the chest (externally or implanted).
Given the small size of rodents, placing the electrode above the targeted cortex on the rodent's skin may lead to excessive shunting, particularly when the counter-electrode is placed in close proximity, e.g., in the neck (for examples of current modeling, see Figure 3 (adopted from reference1)). Additionally, the stability and electrode contact is less reliable and rodents are more irritated by repeated electrode placement on the scalp when using a transdermal application. The fixation of a non-permanent set-up may also hinder the rodent from performing freely. In contrast, the rodents adjust quickly to this permanently present implanted set-up.
The estimation of an equivalent stimulation intensity compared to human stimulation parameters is difficult, since models can only take into account a limited numbers of factors and rodents are pachygyric (see reference1 for estimation of a scaling factor). Therefore, collecting dose-response data including low intensity currents may be most informative. Using the presented surgical set-up in a dose-response study in anesthetized rats, the dose-dependent microglial activation outlasting the stimulation period (24 h post-stimulation) was demonstrated and dissociable from neurodegeneration occurring at high intensity DCS (Figure 4; adopted from reference17). Microglial activation, assessed by morphological alteration, occurred first at 31.8 A/m² (Figure 4C), while the first signs of neurodegeneration were detected at 47.8 A/m². In these experiments, the anesthesia clearly affected the magnitude of response to DCS as the percentage of brain slices with fluorojade C (FJC) positive degenerating neurons in alert rats was higher at 47.8 A/m² (Figure 4A). As the threshold for ≥ 24 h lasting microglial activation is close to the lesion threshold, but greatly above the intensities that promote physiological cognitive and plastic processes in humans, such activation might rather indicate a pre-lesional inflammation induced by DCS. Hence, behavioral or molecular effects of DCS at these high intensities are expected to be mechanistically different compared to low intensity effects (see the scheme summarizing effects and intensities of tDCS experiments in Figure 5).

Figure 1: Supplies for surgery and technical scheme of the tES socket and electrode cap unit. (A) 1. Cotton swabs, 2. disinfectant, 3. stereotactic frame, 4. soldering iron, 5. analgesic (e.g., carprofen), 6. & 7. anesthetics (e.g., xylazine & ketamine), 8. syringe, 9. clipper, 10. ear puncher, 11. eye ointment (e.g., bepanthene), 12. lead-free tin-solder, 13. two-component dental acrylic cement (DAC), 14. iodine, 15. 3% H2O2, 16. 0.9% saline, 17. synthetic braided non-absorbable suture (e.g., Mersilene 4-0), 18. drill bits, 19. cyanoacrylic glue, 20. bulldog clamps 21. homeostatic forceps, 22. bent, serrated tip forceps, 23. straight, sharp tip forceps, 24. straight, tissue forceps, 25. dental spatula, 26. scalpel, 27. scissors, 28. hand drill, 29. screw driver, 30. motor driven drill, 31. female connectors, 32. tES socket, 33. square platinum electrode attached to cable, soldering joint covered with histoacrylic glue, 34. plastic screws. (B) tES socket (red) for fixation on the rodent skull with an inner diameter of 4 mm; the electrode unit (grey) is built by a screw cap and an inner stamp with a center hole leaving room for the cable of the Ag/Cl disc electrode, which is glued to the bottom of the stamp. This allows for maximal stability of the set-up and avoidance of wire breaks at the sensitive wire-electrode-connection point. Please click here to view a larger version of this figure.

Figure 2: Rats equipped with tES set-up. The rat on the left is equipped with an externally fixed counter electrode on the shaved chest. The carbon rubber electrode is sewed to the bottom of the vest. The rat on the right has an implanted chest electrode with the cable tunneled to the head. The female connector attached to the cable (caudal) is fixed within the dental acrylic cement built unit, behind the socket for the tES electrode (rostral). Please click here to view a larger version of this figure.
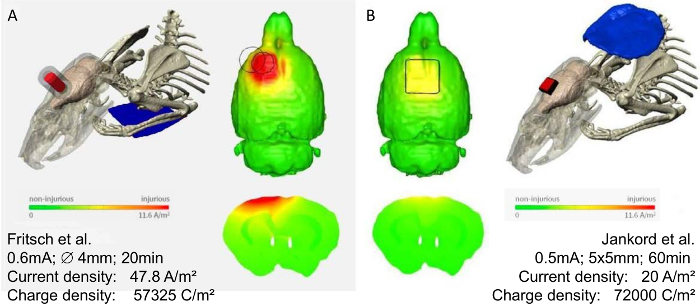
Figure 3: Modeling of current distribution in two different rodent tES montages. Finite element models predicting the brain current flow in two rat models with epicranial tDCS montages, modified with permission1. Using these models, the predicted threshold brain current density to induce cortical lesions was 17.0 A/m² for the montage used by Fritsch, et al.8 (A), and 6.3 A/m² for the montage by Rohan, et al.21 (B), corresponding to electric fields of 61, and 23 V/m, respectively. Note the discrepancies in the current flow pattern of the two different montages. In (A) a higher current density for a shorter time is applied, resulting in a lower charge density than in (B). Most importantly the placement of the counter electrode (neck vs. chest) might have an additional impact on the resulting brain current flow. Therefore, for interpretation of rodent data, the specification of electrode size, placement (both electrodes), applied current, and stimulation duration is necessary. Note that the actual current within the rat brain can only be estimated by using these computational models. Color scale indicates current density from zero to 11.6 A/m², and above. Please click here to view a larger version of this figure.
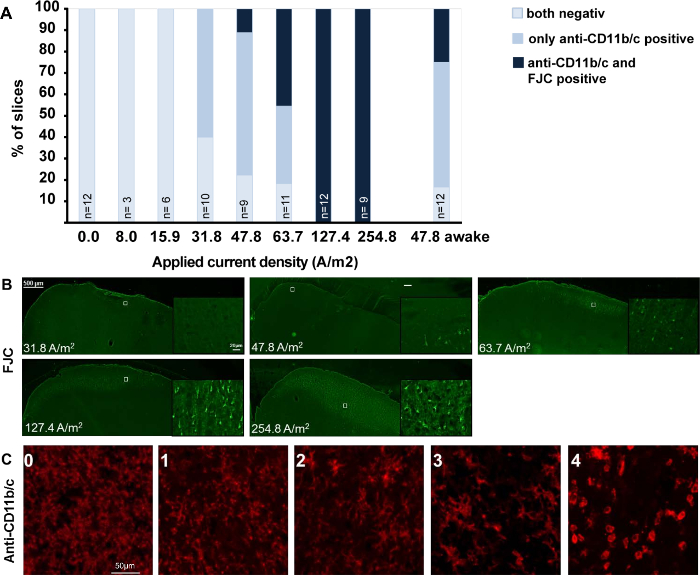
Figure 4: Dose response effects of DCS on microglia activation and neurodegeneration in brain slices obtained after different doses of anodal tDCS applied to the primary motor cortex. The figure is modified from17 summarizing the immunohistological findings of a dose-response tDCS study. (A) Relation between morphologically activated microglia assessed by anti-CD11b/c staining (rating see below) and neurodegeneration revealed by FJC positivity. Rat motor cortical brain slices were rated by a blinded investigator either as anti-CD11b/c and FJC staining negative, as anti-CD11b/c positive only (detection of activation determined morphologically), or as both anti-CD11b/c and FJC positive. Note that microglial activation preceded occurrence of neurodegeneration. (B) Representative coronal sections of left motor cortical brain slices (at or near +1.56 mm from the bregma) from rats exposed to different intensities of anodal tDCS applied to the primary motor cortex. In anesthetized rats, no signs of neurodegeneration occurred at 31.8 A/m², while a few degenerating neurons were present at 47.8 A/m² and neuronal damage further increased with increasing dose. Of note, anodal DCS at 47.8 A/m² in alert rats increased the percentage of slices with neurodegeneration. Scale bar for all sections: 500 µm. Magnification inlet scale bar for all sections: 20 µm. (C) Histological sample images of the rating of microglia activation in anti-CD11b/c immunohistochemistry, ranging from 0 (not activated) to 4 (severely activated), 1-4 were rated as "positive". Scale bars = 50 µm. Please click here to view a larger version of this figure.
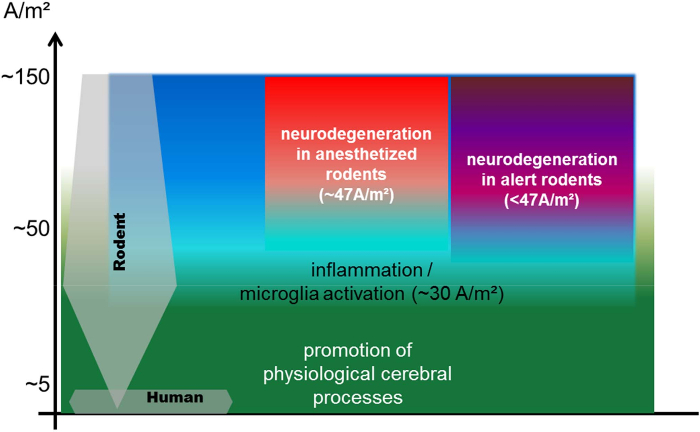
Figure 5: Scheme illustrating the relation of currently used rodent tDCS current densities compared to the human application: thresholds for inflammation, neurodegeneration, and modulation of physiological processes at stimulation durations of up to 30 min. The currently used rodent DCS current densities range from 1.3 to 143 A/m² with the majority of studies using more than 20 A/m², while the current densities in the majority of human studies are between 0.3 and 0.8 A/m²1,14. Human stimulation parameters are at least one order of magnitude below the threshold for neurodegeneration1. Threshold for neurodegeneration is significantly higher under anesthesia, when cortical excitability is suppressed17. Lasting microglial activation begins below but close to the intensities inducing neuronal damage17. Investigation of modulating effects of DCS on physiological cerebral processes at higher intensities below the lesion threshold are likely different from theses seen at very low intensities (comparable to the human application). The exact translation of stimulation parameters between species is under investigation. Estimations are hindered by passive factors like size and anatomy (sulci and gyri), but also by possible different sensitivities to electric fields of neurons, glia, and networks across species (it is not known whether the same current flow would lead to the same physiological effect). Therefore, the most informative study design is testing tES effects in a dose response manner, including very low current intensities. The scheme is based on data from7,12,16,18,19,20,21,22,23,24,25,26,27,28,29,30,31 (maximum stimulation duration of 30 min per session, data are from disease animal models excluded). Please click here to view a larger version of this figure.
Discussion
This protocol describes typical materials and procedural steps for surgical realization of a permanent tES set-up, as well as for subsequent stimulation in alert rodents. During preparation of a rodent tES experiment, several methodological aspects (safety and tolerability of tES, outcome parameter) as well as conceptual aspects (comparability with human condition, anticipated effects of stimulation on a particular brain region) need to be taken into account. From a methodological point of view, the surgical set-up of the cranial tES socket with the implanted chest counter electrode is advantageous for longitudinal studies since it allows for application in alert, freely moving rodents. Habituation of the rodent to the cable connections and permanent set-up is needed, but afterwards, it allows tES even in combination with behavioral tasks. Moreover, the discomfort of the rodent during stimulation is likely lower with this set-up compared to wearing a counter electrode sewn into a vest, since movements are not restricted in the former case and tissue contact of the implanted electrode during stimulation is ensured.
The presented tES set-up has been used for experiments lasting up to 3 months, without any electrode cable discontinuity, material instability, or infection. It is thus likely that the set-up is also suited for experiments longer than this period.
This epicranial stimulation montage prevents excessive shunting via dermal structures, which is likely occurring to a larger extent in rodents given the relation of electrode to body size (see Figure 3 and reference1). The implanted set-up for epicranial stimulation in rodents allows for high practicality for experiments in freely moving animals, a clear definition of electrode positions, and reliability of current delivery. While these factors represent major advantages for the standardization of experiments, the discrepancy to transdermal stimulation in humans or rodents (e.g., references32,33) should be kept in mind. However, for translation of results, the actual amount of current reaching the brain (brain current density), independent of the passage through different tissues, is of major relevance34. Placement of the counter electrode generally determines the direction and distribution of the current flow1. Thus, for polarization of neurons, a placement of the counter electrode on the chest (dorsoventral direction of current flow) may be more effective compared to placement in the neck (rostrocaudal direction of current flow).
Studies addressing the underlying mechanisms of tES or its effect on behavior should whenever feasible be performed in alert rodents, since anesthesia significantly interacts with (patho)-physiological processes including neuronal activity35,36 and plasticity36,37, and may suppress or alter tES efficacy17, (and our unpublished observations) or harm1,17. Vice versa, results obtained in anesthetized rodents do not allow a direct inference to the alert condition, potentially hindering forward translation to research in humans. An example of the differences of tDCS applied to the motor cortex in anesthetized rats and alert rats is shown in the representative results section. tDCS applied at equally high current densities induced a greater rate of microglia activation and neurodegeneration in alert rats compared to anesthetized rats17. While these findings are part of a dose-response experiment in which tDCS effects on microglia and neurodegeneration occurred at high intensities (not recommend for use in mechanistical or behavioral studies), it is tempting to speculate that at low tDCS current densities, the same diversion of results would occur depending on the alertness of the rodent.
Finally, another advantage of the provided protocol is the reduction of possible sources of error. Troubleshooting strategies and critical steps have been highlighted in the protocol. These include the choice of appropriate current density for the particular research purpose (as discussed above), the need for preparation of the tES electrodes before each applied stimulation, and the thoroughly and repeated check for reliable conductance. With regard to electrode preparation, chlorination of the electrode before cathodal stimulation (i.e., cathode over the cortical target area) is absolutely crucial since exhaustion of chlorination during stimulation will lead to toxic build-up by electrochemical reactions and secondarily cause tissue damage. In our experience, exhaustion of chlorination does not occur within the first 20 min of stimulation. On the contrary, before repeated anodal stimulation, Ag/Cl deposition at the anode must be removed to avoid discontinuation of the stimulation. Conductance is a critical point, which does not only relate to the electrode preparation but also to the practical stimulation session of the rodent, as well as the quality of the surgical implantation of the tES socket. Closure of the tES socket to the surrounding skull and tissue area should be ensured during the surgical procedures for correct estimation of the current density. Shunting along adjacent tissue leads to overestimation of the current applied to the brain on one hand, and may alter physiological outcomes based on distortion of the current flow direction. On the other hand, reduction of the stimulation area — either by Ag/Cl deposition (see above) or by coverage of the stimulation area during tES socket implantation (e.g., by acrylic cement or histoacrylic glue) — may lead to underestimation of the applied current density, and stimulation via a reduced area may lead to tissue damage simply by unintentionally high peak current densities. Lastly, the conductive medium, e.g., saline, needs to be optimally distributed over the stimulation area to allow for continued stimulation. During an ongoing experiment, altered conductance may become visible as the following: unexpected behavior of the rodent (signs of stress, variable performance on a particular task), variations in current delivery displayed on an ampere meter of the electrical circuit, or complete loss of stimulation continuity. In this case, the electrodes should be cleaned from depositions and the stimulation area should be inspected again for any debris. Cable connections, placement of tES electrode in the respective socket, and the conductive medium should be checked and replaced when appearing dysfunctional. If tES delivery was inconsistent, the obtained data (e.g., behavior, histology) of this particular session may need to be excluded from analysis.
The threshold for neurodegeneration determined in alert rats (our unpublished data) of both cathodal and anodal sessions are safe and well tolerated in alert rodents, when using up to 20 min of continuous stimulation at intensities below 31.8 A/m² (equivalent to 0.4 mA with a 4 mm diameter transcranial electrode), and when adhering to the recommendations outlined in this protocol. However, choosing lower intensities closer to the human stimulation parameters (0.3-1.6 A/m²) or preferably dose-response experiments are recommended to maximize the informative character of the obtained data and to facilitate forward translation of the results to human application.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the German Research Foundation (DFG RE 2740/3-1). We thank Frank Huethe and Thomas Günther for the in-house production of the custom-made tES set-up and DC-stimulator.
Materials
| Softasept N | B. Braun Melsungen AG, Melsungen, Deutschland |
3887138 | antiseptic agent |
| Ethanol 70 % | Carl Roth GmbH & Co. KG, Karlsruhe, Deutschland | T913.1 | |
| arched tip forceps | FST Fine science tools, Heidelberg, Deutschland | 11071-10 | |
| Iris Forceps, 10cm, Straight, Serrated | World Precision Instruments, Inc, Sarasota, FL, USA, Inc, Sarasota, FL, USA | 15914 | |
| Scalpel Handle #3, 13cm | World Precision Instruments, Inc, Sarasota, FL, USA, Inc, Sarasota, FL, USA | 500236 | |
| Standard Scalpel Blade #10 | World Precision Instruments, Inc, Sarasota, FL, USA, Inc, Sarasota, FL, USA | 500239 | |
| Zelletten cellulose swabs | Lohmann und Rauscher, Neuwied, Deutschland | 13349 | 5 x 4 cm |
| Isoflurane | AbbVie Deutschland GmbH & Co | N01AB06 | |
| Iris Scissors, 11.5cm, Straight | World Precision Instruments, Inc, Sarasota, FL, USA, Inc, Sarasota, FL, USA | 501758 | small scissors |
| cotton swab/cotton buds | Carl Roth GmbH & Co. KG, Karlsruhe, Deutschland | EH12.1 | Rotilabo |
| Kelly Hemostatic Forceps, 14cm, Straight | World Precision Instruments, Inc, Sarasota, FL, USA, Inc, Sarasota, FL, USA | 501241 | surgical clamp |
| electrode plate (platinum) | custom made | Wissenschaftliche Werkstatt Neurozentrum Uniklinik Freiburg, Deutschland | 10×6 mm, 0.15 mm thickness |
| insulated copper strands (~1 mm diameter) | Reichelt elektronik GmbH & Co. KG, Sande, Germany | LITZE BL | electrode cable |
| Weller EC 2002 M soldering station | Weller Tools GmbH, Besigheim, Germany | EC2002M1D | |
| Iso-Core EL 0,5 mm | FELDER GMBH Löttechnik, Oberhausen, Deutschland | 20970510 | lead free solder |
| MERSILENE Polyester Fiber Suture | Johnson & Johnson Medical GmbH, Ethicon Deutschland, Norderstedt, Germany | R871H | nonabsorbable braided suture, 4-0 |
| Histoacryl | B. Braun Melsungen AG, Melsungen, Deutschland |
9381104 | cyanoacrylate |
| Ketamin 10% | Medistar GmbH, Germany | n/a | anesthetics |
| Rompun 2% (Xylazine) | Bayer GmbH, Germany | n/a | anesthetics |
References
- Bikson, M., et al. Safety of Transcranial Direct Current Stimulation: Evidence Based Update 2016. Brain Stimul. 9 (5), 641-661 (2016).
- Bindman, L. J., Lippold, O. C., Redfearn, J. W. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 172, 369-382 (1964).
- Gartside, I. B. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: role of protein synthesis. Nature. 220 (5165), 382-383 (1968).
- Purpura, D. P., McMurtry, J. G. Intracellular activities and potential changes during polarization of motor cortex. Neurophysiol. 28 (1), 166-185 (1965).
- Nitsche, M., Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 527 (Pt 3), 633-639 (2000).
- Nitsche, M. A., Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 57 (10), 1899-1901 (2001).
- Cambiaghi, M., et al. Brain transcranial direct current stimulation modulates motor excitability in mice. Eur J Neuro. 31 (4), 704-709 (2010).
- Fritsch, B., et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 66 (2), 198-204 (2010).
- Ranieri, F., et al. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 107 (7), 1868-1880 (2012).
- Kronberg, G., Bridi, M., Abel, T., Bikson, M., Parra, L. C. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul. 10 (November), 51-58 (2016).
- Sun, Y., et al. Direct current stimulation induces mGluR5-dependent neocortical plasticity. Ann Neurol. 80 (2), 233-246 (2016).
- Podda, M. V., et al. Anodal transcranial direct current stimulation boosts synaptic plasticity and memory in mice via epigenetic regulation of Bdnf expression. Sci Rep. 6, 22180 (2016).
- Reis, J., Fritsch, B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr opin Neurology. 24 (6), 590-596 (2011).
- Buch, E. R., et al. Effects of tDCS on motor learning and memory formation a consensus and critical position paper. Clin Neurophysiol. 128 (4), 589-603 (2017).
- Reis, J., Fischer, J. T., Prichard, G., Weiller, C., Cohen, L. G., Fritsch, B. Time- but not sleep-dependent consolidation of tDCS-enhanced visuomotor skills. Cerebral cortex. 25 (1), 109-117 (2015).
- Monai, H., et al. Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nature Comm. 7, 11100 (2016).
- Gellner, A. -. K., Reis, J., Fritsch, B. Glia: A Neglected Player in Non-invasive Direct Current Brain Stimulation. Front Cell Neurosci. 10, 188 (2016).
- Takano, Y., Yokawa, T., Masuda, A., Niimi, J., Tanaka, S., Hironaka, N. A rat model for measuring the effectiveness of transcranial direct current stimulation using fMRI. Neurosci Lett. 491 (1), 40-43 (2011).
- Islam, N., Moriwaki, A., Hattori, Y., Hori, Y. Anodal polarization induces protein kinase C gamma (PKC gamma)-like immunoreactivity in the rat cerebral cortex. Neurosci Res. 21, 169-172 (1994).
- Islam, N., Aftabuddin, M., Moriwaki, A., Hattori, Y., Hori, Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 684 (2), 206-208 (1995).
- Rohan, J. G., Carhuatanta, K. A., McInturf, S. M., Miklasevich, M. K., Jankord, R. Modulating Hippocampal Plasticity with In Vivo Brain Stimulation. J Neurosci. 35 (37), 12824-12832 (2015).
- Wachter, D., et al. Transcranial direct current stimulation induces polarity-specific changes of cortical blood perfusion in the rat. Exp Neurol. 227 (2), 322-327 (2011).
- Koo, H., et al. After-effects of anodal transcranial direct current stimulation on the excitability of the motor cortex in rats. Rest Neurol Neurosci. 34 (5), 859-868 (2016).
- Liebetanz, D., et al. After-effects of transcranial direct current stimulation (tDCS) on cortical spreading depression. Neurosci Lett. 398 (1-2), 85-90 (2006).
- Fregni, F., et al. Effects of transcranial direct current stimulation coupled with repetitive electrical stimulation on cortical spreading depression. Exp Neurol. 204 (1), 462-466 (2007).
- Cambiaghi, M., et al. Flash visual evoked potentials in mice can be modulated by transcranial direct current stimulation. Neurosci. 185, 161-165 (2011).
- Dockery, C. A., Liebetanz, D., Birbaumer, N., Malinowska, M., Wesierska, M. J. Cumulative benefits of frontal transcranial direct current stimulation on visuospatial working memory training and skill learning in rats. Neurobiol Learn Mem. 96 (3), 452-460 (2011).
- Faraji, J., Gomez-Palacio-Schjetnan, A., Luczak, A., Metz, G. A. Beyond the silence: Bilateral somatosensory stimulation enhances skilled movement quality and neural density in intact behaving rats. Behav Brain Res. 253, 78-89 (2013).
- Pikhovych, A., et al. Transcranial Direct Current Stimulation Modulates Neurogenesis and Microglia Activation in the Mouse Brain. Stem Cells In. , 1-10 (2016).
- Rueger, M. A., et al. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PloS one. 7 (8), e43776 (2012).
- Liebetanz, D., Koch, R., Mayenfels, S., König, F., Paulus, W., Nitsche, M. A. Safety limits of cathodal transcranial direct current stimulation in rats. Clinical Neurophysiol. 120 (6), 1161-1167 (2009).
- Yoon, K. J., Oh, B. -. M., Kim, D. -. Y. Functional improvement and neuroplastic effects of anodal transcranial direct current stimulation (tDCS) delivered 1 day vs. 1 week after cerebral ischemia in rats. Brain Res. 1452, 61-72 (2012).
- Spezia Adachi, L. N., et al. Exogenously induced brain activation regulates neuronal activity by top-down modulation: conceptualized model for electrical brain stimulation. Exp Brain Res. 233 (5), 1377-1389 (2015).
- Jackson, M. P., et al. Safety parameter considerations of anodal transcranial Direct Current Stimulation in rats. Brain, behavior, and immunity. , (2017).
- Ordek, G., Groth, J. D., Sahin, M. Differential effects of ketamine/xylazine anesthesia on the cerebral and cerebellar cortical activities in the rat. J Neurophysiol. 109 (5), 1435-1443 (2013).
- Sykes, M., et al. Differences in Motor Evoked Potentials Induced in Rats by Transcranial Magnetic Stimulation under Two Separate Anesthetics: Implications for Plasticity Studies. Front Neural Circ. 10, 80 (2016).
- Zhang, D. X., Levy, W. B. Ketamine blocks the induction of LTP at the lateral entorhinal cortex-dentate gyrus synapses. Brain Res. 593 (1), 124-127 (1992).

