In Situ High Pressure Hydrogen Tribological Testing of Common Polymer Materials Used in the Hydrogen Delivery Infrastructure
Summary
A test methodology for quantifying tribological properties of polymers used in hydrogen infrastructure service is demonstrated and characteristic results for a common elastomer are discussed.
Abstract
High pressure hydrogen gas is known to adversely affect metallic components of compressors, valves, hoses, and actuators. However, relatively little is known about the effects of high pressure hydrogen on the polymer sealing and barrier materials also found within these components. More study is required in order to determine the compatibility of common polymer materials found in the components of the hydrogen fuel delivery infrastructure with high pressure hydrogen. As a result, it is important to consider the changes in physical properties such as friction and wear in situ while the polymer is exposed to high pressure hydrogen. In this protocol, we present a method for testing the friction and wear properties of ethylene propylene diene monomer (EPDM) elastomer samples in a 28 MPa high pressure hydrogen environment using a custom-built in situ pin-on-flat linear reciprocating tribometer. Representative results from this testing are presented which indicate that the coefficient of friction between the EPDM sample coupon and steel counter surface is increased in high pressure hydrogen as compared to the coefficient of friction similarly measured in ambient air.
Introduction
In the recent years, there has been great interest in hydrogen as a potential zero emission or near-zero emission fuel in vehicles and stationary power sources. Since hydrogen exists as a low density gas at room temperature, most applications use some form of compressed hydrogen for fuel.1,2 A potential drawback of using compressed, high pressure hydrogen gas is incompatibility with many materials found within infrastructure2,3,4 and vehicular applications5 where compatibility issues are combined with repeated pressure and temperature cycling. A pure hydrogen environment is known to damage metal components including certain steels and titanium through different mechanisms including hydride formation, swelling, surface blistering, and embrittlement.2,6,7,8 Non-metallic components such as lead zirconate titanate (PZT) used in piezoelectric ceramics have also proven susceptible to degradation due to hydrogen incompatibility effect such as surface blistering and lead migration.9,10,11,12 While these examples of damage due to hydrogen exposure have been previously studied, the compatibility of polymer components within hydrogen environments has only recently become of interest.13,14,15,16 This is largely a result of metallic components providing structural integrity in nuclear and oil and gas applications whereas the polymer components usually act as barriers or seals.17,18,19,20 As a result, the friction and wear properties of polymer materials within components such as polytetrafluoroethylene (PTFE) valve seats and nitrile butadiene rubber (NBR) O-rings become important factors in their ability to function.
In the case of the hydrogen infrastructure, components such as valves, compressors, and storage tanks contain polymer materials that are in contact with metallic surfaces. The frictional interaction between the polymer and metallic surfaces results in wear of each of the surfaces. The science of the relationship between the friction and wear of two interacting surfaces is known as tribology. Polymers tend to have lower elastic moduli and strength than metallic, therefore the tribological properties of polymer materials differ greatly from metallic materials. As a result, polymer surfaces tend to exhibit greater wear and damage after frictional contact with a metallic surface.21,22 In a hydrogen infrastructure application, rapid pressure and temperature cycling causes repeated interaction between the polymer and metallic surfaces, increasing the likelihood of friction and wear on the polymer component. Quantifying this damage can be challenging ex situ due to possible explosive decompression of the polymer sample after depressurization which may cause non-tribological damage.23 Additionally, many commercial polymer products contain many fillers and additives such as magnesium oxide (MgO) that may interact negatively with hydrogen gas through hydriding, further complicating ex situ analysis of wear in these materials.24,25
Due to the complexity of differentiating between damage to the polymer material caused during depressurization and damage due to tribological wear ex situ, there is a need to directly study the frictional properties of non-metallic materials in situ within a high-pressure hydrogen environment that is likely to exist within the hydrogen delivery infrastructure. In this protocol, we demonstrate a test methodology developed to quantify the friction and wear properties of polymer materials in a high-pressure hydrogen environment utilizing a purpose-built in situ tribometer.26 We also present representative data acquired using the in situ tribometer and ethylene propylene diene monomer (EPDM) rubber, a common polymer sealing and barrier material. The EPDM material for which representative data was generated using the protocol below was purchased in 60.96 cm square sheets with a 0.3175 cm thickness and was reported by the vendor to have a 60A hardness rating.
Protocol
The experiment described here requires the use of hydrogen gas which is odorless, colorless, and thus undetectable by human senses. Hydrogen is highly flammable and burns with a nearly invisible blue flame and can form explosive mixtures in the presence of oxygen. High pressures in excess of 6.9 MPa add additional explosion hazards that must be appropriately planned for in preparation for any testing. This amount of stored energy represents a serious safety hazard and therefore due diligence, planning, and a safety evaluation must be performed before performing such an experiment to ensure that these hazards are mitigated. The experiment presented here is performed in accordance with appropriate safety precautions in an American Society of Mechanical Engineers (ASME) certified pressure vessel with a burst disk set to 34.5 MPa with proper ventilation.
1. Preparing Polymer Sheet Stock
- Apply detergent to the EDPM polymer sheet stock using a non-abrasive sponge and rinse under water for approximately 3 min to remove oils and talc powder applied during the manufacturing and shipping process.
- Dry polymer sheet in a drying oven at 85% of the material's working temperature, about 75 °C for EPDM, for approximately 72 hours to drive of any water remaining from washing.
- Turn off the oven and allow polymer sheet stock material to cool to room temperature inside the oven.
- Mark one corner of the sheet stock with an arrow pointing to the top of the polymer sheet. This arrow will assist with identifying orientation of the sheet during the sample coupon generation, ensuring that samples cut from the polymer sheet will be consistently of the same orientation.
- Store the polymer stock sheet in a room temperature, humidity-controlled environment near 25% relative humidity prior to tribological testing.
2. Generating and Mounting Sample Coupons
- While wearing powder free gloves, mark the polymer sheet stock with an arrow in the intended coupon area near the arrow marked during preparation of the polymer sheet stock such that both arrows have the same orientation.
- Using a 2.222 cm diameter circular die and a mallet, stamp out a sample coupon around the arrow mark.
- Loosen the hex cap screws securing the sample clamp on the in situ tribometer, remove the hex cap screw and precision spring from the most easily accessible corner of the sample clamp.
- Slide sample coupon into sample clamp, taking care to make sure the sample is oriented with the arrow pointed face down and towards the back of the clamp which is the side closest to the back-plate of the tribometer.
- Replace the precision spring and hex cap screw in the empty corner of the sample clamp and proceed to hand tighten all four of the hex cap screws of the clamp until snug such that the elastomer sample is compressed by 10% of its original height. Assuming a 0.318 cm sample height, 10% compression can be achieved by using a 0.287 cm gauge block between the two plates of the clamp.
3. Preparing the In Situ Tribometer
- Place a 2.413 cm gauge block between the wall of the tribometer and the sample sled, directly below the drive screw. Make sure the data collection box is turned off, then turn the drive chain in a clockwise motion to back the sample sled such that the edge of the sled is 2.413 cm from the tribometer wall.
- Gently wipe the steel ball of the counter surface with a soft cloth or lint-less paper towel and an appropriate solvent such as acetone for approximately 30 seconds until the surface of the counter surface appears free of any debris.
- Slide the bronze counter surface carrier and bronze weight, a total normal load of 7.5 N, onto the rail perpendicular to the sample sled, allowing the counter ball to slide between into the keyhole and rest on the polymer sample.
- Using a hex key and two bronze screws, reattach the linear variable differential transformer (LVDT) measurement arm to the bronze counter surface holder such that the freely floating cylinder of the LVDT rests on the arm.
- Adjust the clamp holding the LVDT in place up or down such that the LVDT is measuring near it's zero point then tighten the clamp to secure the LVDT in place.
- Lower the tribometer assembly into the pressure vessel, ensuring that the thermowell in the top flange of the vessel will lower into the gap between the tribometer and the wall of the vessel.
- Wrap the sealing o-ring with a total of two and a half layers of PTFE tape. This is accomplished by wrapping the PTFE tape such that each additional wrap overlaps approximately half of the proceeding lap until going around the diameter of the o-ring twice. Then wrap the diameter of the o-ring a final time without any overlap. Once the o-ring is wrapped, place it in the groove in the lip of the pressure vessel.
- Taking into account the wiring labels, reconnect the five power wires for the tribometer motor, four data wires for the load cell, and five data wires for the LVDT.
4. Sealing the Pressure Vessel
- Lower the top flange of the pressure vessel to close it, taking care to lower the top flange gently onto the PTFE wrapped sealing o-ring.
- Insert the bolts into the numbered holes on the top flange indicated by the manufacturer in ascending order until they are finger tight.
- Using a manual hex key, torque the flange bolts in ascending order to hand tight and repeat until the bolts can no longer be tightened.
- Starting at 120 Nm and increasing in ~40 Nm increments, use a torque wrench to torque the flange bolts in ascending order for each ~40 Nm increment until they are torqued to 280 Nm.
5. Filling the Pressure Vessel
- Now that the pressure vessel is sealed, connect the gas fittings to the autoclave lid and flush the pressure vessel with low pressure (~0.55 MPa) argon gas for approximately 1 h until the oxygen content of the vessel drops below 10 ppm using an oxygen sensor plumbed into the output of the pressure vessel.
- Slowly (<0.25 MPa/s) flush the vessel with hydrogen gas up to 6.9 MPa, then slowly vent the gas to atmospheric pressure. Repeat the flushing process two more times.
- After flushing the pressure vessel, slowly (<0.25 MPa/s) fill the pressure vessel with hydrogen gas up to 13.75 MPa and allow the vessel to rest for 10 min such that the temperature of the gas within the vessel equilibrates to room temperature.
- Fill the vessel to 20.7 MPa and wait another 10 min.
- Bring the vessel up to the target 27.6 MPa and close off all valves.
- Allow the polymer sample to soak for at least 12 h in the hydrogen gas before starting the experiment to allow for complete permeation.
6. Running Experiment
- Double check that all the pass-through wires exiting the pressure vessel are correctly connected to the labelled wiring harness attached to the tribometer control box, and then turn on the tribometer.
- In the tribometer software set the experiment time to 1 hour at 0.1 cm/s velocity with a path length of 0.140 cm. This corresponds to a distance of approximately 3.5 m.
- Tare the load cell, and ensure that the LVDT is reporting an appropriate depth in the tribometer software which should be near 0 mm.
- Start the experiment.
7. Post-Experiment
- Once the experiment has completed, slowly vent the pressure vessel of hydrogen gas at approximately 0.35 MPa/s, ensuring that the pressure vessel temperature does not drop below 0 °C.
- Finally, flush the pressure vessel volume with argon gas at atmospheric pressure for 10 minutes to ensure that there is no remaining hydrogen within the vessel.
Representative Results
Using the methodology presented, the coefficient of kinetic friction and wear factor for an elastomeric sample can be measured while in a high-pressure hydrogen environment. The representative data presented in Figure 1 show that in a high-pressure hydrogen environment greater force is required to move EPDM polymer samples under the steel counter surface. Using the relationship between the normal force FN and the frictional force FK the coefficient of friction, µ, between the EPDM sample and the steel ball can be determined. This data is presented in Figure 2 where the EPDM samples exhibit a higher coefficient of friction in hydrogen than samples tested in ambient air. This result indicates that there is more friction occurring due to the sliding contact between the EPDM polymer steel surfaces while in a high-pressure hydrogen environment as compared to ambient air.
Figure 3 reveals that the penetration depth of the steel counter surface into the EPDM polymer samples in high pressure hydrogen is less than the depth measured in ambient air samples. As in previous studies26, the effective wear factor, K*, describes the amount of material removed from the surface can be calculated using Equation 1 from the penetration depth XPD, the contact pressure P, the wear volume V, and the time T. This K* parameter is referred to as an "effective" wear factor because the combination of both removal of material and the deformation of the polymer surface that contribute to a wear depth measured by the LVDT position sensor. Figure 4 shows that the EPDM samples have a lower effective wear factor in high pressure hydrogen by the end of the experiment. This phenomenon is most likely a pressure effect and is not necessarily an indication that wear in hydrogen gas is less than in ambient air conditions.
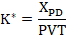
Equation 1: Relationship between the effective wear factor (K*) and the penetration depth (XPD), the contact pressure (P) of the counter-surface on the polymer sample, the volume of the wear track (V), and time (T).
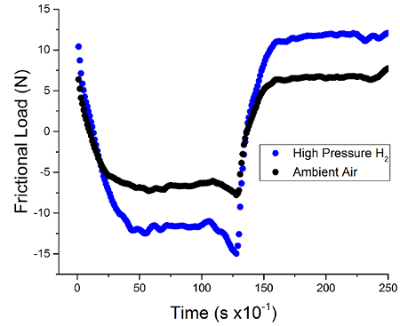
Figure 1: Representative frictional load data acquired using the in situ tribometer's load cell of an EPDM polymer sample coupon at cycle #120 as a function of time. Data acquired in high pressure hydrogen is in blue, and data acquired in ambient air is in black. Please click here to view a larger version of this figure.
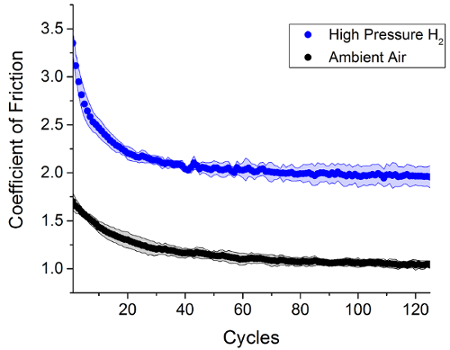
Figure 2: Coefficient of friction data calculated from frictional load data such as in Figure 1. The amount of friction between the EPDM sample and the steel counter surface is much higher in high pressure hydrogen than in ambient air. Please click here to view a larger version of this figure.
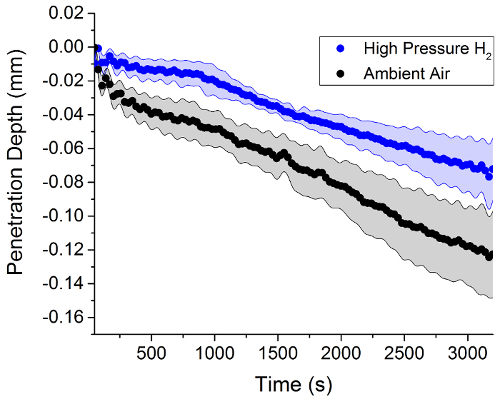
Figure 3: Penetration depth data gathered from the LVDT component of the in situ tribometer from testing on EPDM polymer samples. As in the friction data, the high pressure hydrogen data is blue while the ambient air data is in black. Please click here to view a larger version of this figure.
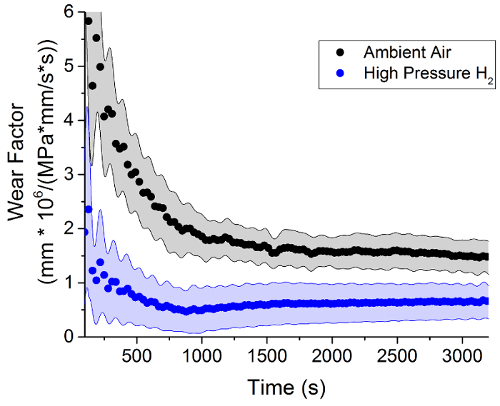
Figure 4: The wear factor calculated from the penetration depth data presented in Figure 3. The wear factor of the ambient air sample is higher than the wear factor of sample tested in high pressure hydrogen, which is most likely a pressure effect. Please click here to view a larger version of this figure.
Discussion
Current ex situ techniques for tribological testing of polymer materials require samples to be exposed to high pressure hydrogen which are then depressurized before being tested using a commercial tribometer.15,24,25 The test methodology in this protocol was designed to permit testing of the tribological properties of polymer samples in a high pressure environment in situ. By testing polymer materials such as the EPDM samples presented above while they are pressurized, this protocol allows for a more realistic measurement of the dense pressure-compressed polymer sample found in components of the hydrogen delivery infrastructure. Since the tribological properties of the material are measured in situ, data anomalies caused by depressurization effects such as explosive decompression present in ex situ methods are mitigated.
This protocol requires adequate sample soak times of the polymer sample attached to the tribometer instrument in order to ensure that the hydrogen gas has completely diffused throughout the polymer sample, which in the case of EPDM was approximately 12 hours. As a result measurement of the polymer sample’s tribological properties while being exposed to high pressure hydrogen gas, the metallic functional and structural components of the tribometer used in this protocol were required to be hydrogen gas compatible. Therefore, the in situ tribometer was mostly constructed out of aluminum and the use of stainless steel was minimized. Functional components such as the motor driving the sample stage and a capacitive load cell used to measure the frictional load in the tribometer constructed using hydrogen-compatible components and were specially ordered for this project. These components increased the cost of performing this in situ methodology as compared to the ex situ alternatives.
The in situ test methodology described here has been developed to quantitatively measure the friction and wear of polymer samples while in a hydrogen gas environment similar to the high pressure conditions that exist within the hydrogen delivery infrastructure. The results of this testing can be used to help determine the suitability of a given polymer material for use in hydrogen infrastructure and storage applications. The data generated using this methodology and presented above for EPDM polymer samples suggests that the surface coefficient friction of these samples along with the wear EPDM samples experienced was increased in a high pressure hydrogen environment. This methodology was not able to determine whether these trends were due to the pressure effects of the in situ environment or the interaction between the hydrogen gas and the EPDM polymer. Future study is required to deconvolute the effects of pressure and hydrogen compatibility in these elastomeric samples with in a high-pressure hydrogen environment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was performed at the Pacific Northwest National Laboratory (PNNL), which is operated by Battelle Memorial Institute for the Department of Energy (DOE) under Contract No. DE-AC05-76RL01830.
Materials
| EPDM Polymer Stock Sheet | McMaster-Carr | 8525T68 | 24" x 24", 1/8" Thick |
| Pressure Vessel, Autoclave | Fluitron Inc. | 8308-1788-U | 5" diameter, 1' height |
| High Purity Hydrogen Gas | Praxair | HY4.5 | Grade 4.5, 5ppm O2, 5 ppm H20 |
| O2 Sensor | Advanced Micro Instruments | T2 | 0-5ppm min. range, 10,0000ppm max. |
| Pre-purified Argon Gas | Oxarc | LCCO-HP818 | High-purity, 99.998% |
| Liquid Dishwashing Detergent | McMaster-Carr | 98365T89 | 32 oz pour bottle, lemon scented |
| Mildew Resistant Sponge | McMaster-Carr | 7309T1 | 6" long x 3 -1/2" Wide x 1" High, yellow |
| PTFE Pipe Thread Sealant Tape | McMaster-Carr | 4591K12 | 1/2" wide, white color |
| Gas Tube Fittings | Swagelok | SS-400-1-4 | 1/4" OD, stainless steel, male NPT threading |
| Hammer Driven Die | McMaster-Carr | 3427A22 | 7/8" Hammer driven hole punch |
| Linear Variable Differential Transformer | Omega | LD320-2.5 | 2.5mm, AC output, guided w/spring |
| Autoclave O-ring Seal | Fluitron Inc. | A-4511 | Hastelloy C-276, 5-3/4" OD x 5" ID x 3/8" |
| Torque Wrench | McMaster-Carr | 85555A422 | Adjustable Torque-Limiting Wrench, Quick-Release, 1/2" Square Drive, 50-250 ft.-lbs. Torque |
| Mallet | McMaster-Carr | 5939A11 | Hard and Extra-Hard Rubber Hammer, 2-1/4 lbs. |
| iLoad Mini Capacitive Load Sensor | Loadstar Sensors | MFM-050-050-S*C03 | 50 lb, U Calibration, 0.5% Accuracy, Steel |
References
- Schlapbach, L. Technology: Hydrogen-fuelled vehicles. Nature. 460 (7257), 809-811 (2009).
- Jones, R., Thomas, G. . Materials for the Hydrogen Economy. , (2007).
- Barth, R., Simons, K. L., San Marchi, C. . Polymers for Hydrogen Infrastructure and Vehicle Fuel Systems: Applications, Properties, and Gap Analysis. , 23-34 (2013).
- Marchi, C., Somerday, B. P., Ref, M. T. . Technical Reference on Hydrogen Compatibility of Materials. , (2008).
- Welch, A., et al. . Challenges in developing hydrogen direct injection technology for internal combustion engines. , (2008).
- Fukai, Y. . The Metal-Hydrogen System. , (2005).
- Lu, G., Kaxiras, E. Hydrogen embrittlement of aluminum: The crucial role of vacancies. Phys. Rev. Lett. 94 (15), 155501 (2005).
- Zhao, Z., Carpenter, M. A. Annealing enhanced hydrogen absorption in nanocrystalline Pd∕AuPd∕Au sensing films. J. Appl. Phys. 97 (12), 124301 (2005).
- Alvine, K. J., et al. High-pressure hydrogen materials compatibility of piezoelectric films. Appl. Phys. Lett. 97 (22), 221911 (2010).
- Alvine, K. J., et al. Hydrogen species motion in piezoelectrics: A quasi-elastic neutron scattering study. J. Appl. Phys. 111 (5), 53505 (2012).
- Aggarwal, S., et al. Effect of hydrogen on Pb(Zr,Ti)O3Pb(Zr,Ti)O3-based ferroelectric capacitors. Appl. Phys. Lett. 73 (14), (1998).
- Ikarashi, N. Analytical transmission electron microscopy of hydrogen-induced degradation in ferroelectric Pb(Zr, Ti)O3Pb(Zr, Ti)O3 on a Pt electrod. Appl. Phys. Lett. 73 (14), (1998).
- Castagnet, S., Grandidier, J., Comyn, M., Benoı, G. Hydrogen influence on the tensile properties of mono and multi-layer polymers for gas distribution. Int. J. Hydrog. Energy. 35, 7633-7640 (2010).
- Theiler, G., Gradt, T. Tribological characteristics of polyimide composites in Hydrogen environment. Tribol. Int. 92, 162-171 (2015).
- Sawae, Y., et al. Friction and wear of bronze filled PTFE and graphite filled PTFE in 40 MPA hydrogen gas. Proceed. , 249-251 (2011).
- Fujiwara, H., Ono, H., Nishimura, S. Degradation behavior of acrylonitrile butadiene rubber after cyclic high-pressure hydrogen exposure. Int. J. Hydrogen Energy. 40 (4), 2025-2034 (2015).
- Zhang, L., et al. Influence of low temperature prestrain on hydrogen gas embrittlement of metastable austenitic stainless steels. Int. J. Hydrogen Energy. 38 (25), 11181-11187 (2013).
- Weber, S., Theisen, W., Martı, M. Development of a stable high-aluminum austenitic stainless steel for hydrogen applications. Int. J. Hydrogen Energy. 38 (14), 5989-6001 (2013).
- Papavinasam, S. . Corrosion control in the oil and gas industry. , (2013).
- Yamamoto, S. Hydrogen Embrittlement of Nuclear Power Plant Materials. Mat. Trans. 45 (8), 2647-2649 (2004).
- Rymuza, Z. Tribology of polymers. Arch. Civ. Mech. Eng. 7 (4), 177-184 (2007).
- Mckeen, L. W. . 1 Introduction to Fatigue and Tribology of Plastics and Elastomers. , (2010).
- Lorge, O., Briscoe, B. J., Dang, P. Gas induced damage in poly(vinylidene fluoride) exposed to decompression. Polymer. 40, 2981-2991 (1999).
- Sawae, Y., Yamaguchi, A., Nakashima, K., Murakami, T., Sugimura, J. Effects of Hydrogen Atmosphere on Wear Behavior of PTFE Sliding Against Austenitic Stainless Steel. Proceed. , 1-3 (2008).
- Sawae, Y., Nakashima, K., Doi, S., Murakami, T., Sugimura, J. Effects of high pressure hydrogen on wear of PTFE and PTFE composite. Proceed. , 233-235 (2010).
- Duranty, E., et al. An in situ tribometer for measuring friction and wear of polymers in a high pressure hydrogen environment. Rev. Sci. Instrum. 88 (9), (2017).

