A Yeast 2-Hybrid Screen in Batch to Compare Protein Interactions
Summary
Batch processing of yeast 2-hybrid screens allows for direct comparison of the interaction profiles of multiple bait proteins with a highly complex set of prey fusion proteins. Here, we describe refined methods, new reagents, and how to implement their use for such screens.
Abstract
Screening for protein-protein interactions using the yeast 2-hybrid assay has long been an effective tool, but its use has largely been limited to the discovery of high-affinity interactors that are highly enriched in the library of interacting candidates. In a traditional format, the yeast 2-hybrid assay can yield too many colonies to analyze when conducted at low stringency where low affinity interactors might be found. Moreover, without a comprehensive and complete interrogation of the same library against different bait plasmids, a comparative analysis cannot be achieved. Although some of these problems can be addressed using arrayed prey libraries, the cost and infrastructure required to operate such screens can be prohibitive. As an alternative, we have adapted the yeast 2-hybrid assay to simultaneously uncover dozens of transient and static protein interactions within a single screen utilizing a strategy termed DEEPN (Dynamic Enrichment for Evaluation of Protein Networks), which incorporates high-throughput DNA sequencing and computation to follow the evolution of a population of plasmids that encode interacting partners. Here, we describe customized reagents and protocols that allow a DEEPN screen to be executed easily and cost-effectively.
Introduction
A complete understanding of cell biological processes relies on finding the protein interaction networks that underlie their molecular mechanisms. One approach to identify protein interactions is the yeast 2-hybrid (Y2H) assay, which works by assembling a functioning chimeric transcription factor once two protein domains of interest bind to one another1. A typical Y2H screen is performed by creating a population of yeast that houses both a library of plasmids encoding interacting proteins fused to a transcriptional activator (e.g., 'prey' fusion protein) and a given 'bait' plasmid comprised of the protein of interest fused to a DNA binding domain (e.g., the Gal4 DNA-binding domain that binds to the Gal4-upstream activating sequence). One of the main advantages of the Y2H approach is that it is relatively easy and inexpensive to conduct in a typical laboratory equipped for routine molecular biological work2. However, when traditionally performed, a user samples individual colonies that arise upon selection for a positive Y2H interaction. This severely limits the number of library 'prey' clones that can be surveyed. This problem is compounded when the abundance of a particular interacting prey is very high relative to the others, diminishing the chance of detecting interaction from low abundance prey plasmids.
One solution for using the Y2H principle in comprehensive coverage of the proteome is the use of a matrix-formatted approach wherein an array containing known individual prey plasmids can be digitally interrogated. However, such an approach requires an infrastructure that is not readily accessible or cost-effective to individual investigators who are interested in defining the interactome of a small number of proteins or domains3. In addition, very complex prey libraries that may encode multiple fragments of interacting proteins would expand the size of such matrix arrays to impractical sizes. An alternative is to perform assays with complex libraries in batches and assess the presence of interacting clones using massive parallel high-throughput sequencing4. This can be applied to assay the presence of prey plasmids that arise in multiple colonies using a typical Y2H formatted approach in which yeast cells housing an interacting pair of fusion proteins are allowed to grow on a plate5,6. This general idea can be accentuated to increase query of both multiple bait and prey components at the same time7,8.
Still, many investigations require an easier yet more focused effort on just a few protein 'baits' and can benefit more by an exhaustive and semi-quantitative query of a single complex prey library. We have developed and validated an approach to perform wide-scale protein interaction studies using a Y2H principle in batch format4. This uses the rate of expansion of a particular prey plasmid as a proxy for the relative strength of Y2H interaction9. Deep sequencing of all plasmids within a population subjected to normal growth or selective growth conditions produces a complete map of clones that yield strong and weak Y2H interactions. The repertoire of interactors can be obtained and directly compared across multiple bait plasmids. The resulting workflow termed DEEPN (Dynamic Enrichment for Evaluation of Protein Networks) can thus be used to identify differential interactomes from the same prey libraries to identify proteins, allowing comparison between one protein vs. another.
Here, we demonstrate DEEPN and introduce improvements in the laboratory methods that facilitate its use, which are outlined in Figure 1. Significant improvements include:
Generation of prey yeast populations. One of the key requirements of DEEPN is generating populations of yeast with different bait plasmids that have the same distribution of the plasmid prey libraries. Equivalent baseline populations of the prey plasmid library are essential for making accurate comparisons between the interactomes of different baits. This is best achieved when a library plasmid is already housed in a haploid yeast population and moving a given bait plasmid into that population is achieved by mating to produce a diploid. Here, we provide a clear guide in how to make such populations using commercial libraries housed in haploid yeast. Although we found methods that generate a high number of diploids, the overall mating efficiency of these commercial library-containing yeast strains was low. Therefore, we constructed a new strain that can house prey libraries that yields far more diploids per mating reaction.
New set of bait plasmids. Many current plasmids that express 'bait' fusion proteins comprised of the protein of interest and a DNA-binding domain are 2µ-based, allowing them to amplify their copy number. This copy number can be quite variable in the population and lead to variability in the Y2H transcriptional response. This in turn could skew the ability to gauge the strength of a given protein interaction based on the growth response of cells under selection. This can be partly address by using a low copy plasmid, some of which have been previously described such as the commercially available pDEST3210. We constructed a new bait plasmid (pTEF-GBD) that produces Gal4-DNA-binding domain fusion proteins within a TRP1 centromere-based low copy plasmid carrying the Kanr resistance gene that also allows cloning of bait fragments both upstream and downstream of the Gal4 DNA-binding domain.
New High-Density Y2H fragment library. We constructed a new plasmid to house Y2H prey libraries and used it to build a highly complex Y2H library made of randomly sheared fragments of genomic DNA from Saccharomyces cerevisiae. Sequence analysis showed that this library had over 1 million different elements, far more complex than previously described yeast genomic Y2H plasmid libraries11. With this new library, we were able to show that the DEEPN workflow is robust enough to accommodate complex libraries with many different plasmids in a manner that is reliable and reproducible.
Protocol
1. Preparation of Media and Plates
NOTE: All plates need to be made minimally 2 days before beginning the protocol. The media can be made at any point. However, the buffered yeast extract peptone dextrose adenine (bYPDA) needs to be made the day of which it will be used. Some media is made using a supplement mix containing a level of adenine that is larger than what is typically used. Most minimal media supplements specify 10 mg/L adenine. Supplements labeled '+40Ade' specify a total of 40 mg/L adenine.
- Prepare Glucose Solution (50% w/v). For 1 L, dissolve 500 g of D-(+)-Glucose in 800 mL of distilled water in a 1,000 mL beaker. Adjust volume to 1,000 mL of using a graduated cylinder and filter through a 0.2 µm sterile filter into a sterile 1,000 mL media storage bottle.
- Prepare Yeast extract peptone dextrose (YPD) plates. For 1 L, dissolve 20 g of Peptone and 10 g of Yeast Extract in 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder, fill up to 960 mL with distilled water. Pour into a 2,000 mL Erlenmeyer flask and add 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Use a pipette to add 40 mL of 50% glucose. Mix well by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare Complete synthetic minimal media (CSM)-Trp plates. For 1 L, dissolve 6.7 g of Yeast Nitrogen Base without amino acids into 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder, fill up to 960 mL of with distilled water. Pour into a 2,000 mL of Erlenmeyer flask and add 0.7 g of -Trp -Met dropout mix, 20 mg of methionine, and 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Use a pipette to add 40 mL of 50% glucose. Mix well by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare CSM-Leu-Met plates. For 1 L, dissolve 10.05 g of Yeast Nitrogen Base without amino acids into 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder and fill up to 940 mL of with distilled water. Pour into a 2,000 mL of Erlenmeyer flask and add 1.005 g of -Leu -Met dropout mix and 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Use a pipette to add 60 mL of 50% glucose. Mix well by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare CSM-Leu-Trp plates. For 1 L, dissolve 10.05 g of Yeast Nitrogen Base without amino acids into 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder, fill up to 940 mL of with distilled water. Pour into a 2,000 mL of Erlenmeyer flask and add 1.005 g of -Trp -Leu+40Ade dropout mix, 240 mg of adenine, and 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Use a pipette to add 60 mL of 50% glucose. Mix well by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare CSM-Leu-Trp-His plates. For 1 L, dissolve 10.05 g of Yeast Nitrogen Base without amino acids into 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder, fill up to 940 mL of with distilled water. Pour into a 2,000 mL of Erlenmeyer flask and add 0.975 g of -Trp -Leu-His+40Ade dropout mix, 240 mg of adenine, and 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Use a pipette to add 60 mL of 50% glucose. Mix well by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare CSM-Leu-Trp-His-3AT plates. For 1 L, dissolve 10.05 g of Yeast Nitrogen Base without amino acids into 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder, fill up to 940 mL of with distilled water. Pour into a 2,000 mL of Erlenmeyer flask and add 0.975 g of -Trp -Leu-His+40Ade dropout mix, 240 mg of adenine, and 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Use a pipette to add 60 mL of 50% glucose. Mix in 100 µL of a 1 M sterile stock of 3-amino-1,2,4 triazole (3AT) by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare LB-Kanr plates. For 1 L, dissolve 10 g of Tryptone, 5 g of Yeast extract and 10 g of NaCl in 800 mL of distilled water in a 1,000 mL beaker. Pour into a graduated cylinder, fill up to 1,000 mL of with distilled water. Pour into a 2,000 mL of Erlenmeyer flask and add 15 g of agar. Autoclave and cool in 37 °C water bath until water bath temperature has cooled to approximately 42 – 50 °C. Add 50 mg Kanamycin and mix by swirling.
- Pour a series of 100 mm plates with 20 mL of media by pipette.
- Prepare YPD, CSM-Leu-Met, CSM-Trp, CSM-Leu-Trp and CSM-Leu-Trp-His media. Use the procedure above for plates except instead of pouring into an Erlenmeyer flask, pour into a media storage bottle and omit the agar.
- Prepare bYPDA (buffered YPDA). Take sterile YPD media and add 200 mg/L adenine in sterile distilled water. Adjust pH to 3.7 with HCl. Filter through a 0.2 µm sterile filter into a sterile bottle.
- Prepare Transformation Buffer: 2 M sorbitol, 1 M lithium acetate dihydrate, 10 mM Tris pH 7.6, 0.5 mM EDTA, 0.2 mM calcium chloride in distilled water. Filter through a 0.2 µm sterile filter into a sterile bottle.
- Prepare PEG solution: 70% w/v polyethylene glycol 3350 in distilled water. Sterilize by autoclave.
- Prepare Twirl: 8 M urea, 4% w/v SDS, 50 mM Tris pH 6.8, 10% v/v glycerol, 0.02% w/v bromophenol blue in sterile distilled water.
- Prepare sTE (strong TE): 50 mM Tris, 20 mM EDTA, pH 8.0 in distilled water. Filter through a 0.2 µm sterile filter into a sterile bottle.
- Prepare Zymolase stock solution: 10 mg/mL Zymolase 100T in 50 mM potassium phosphate dibasic pH 7.5, 50% v/v glycerol buffer in sterile distilled water (stored at -20 °C).
2. Cloning and Verification of Bait Plasmids
NOTE: Construction of Gal4-DNA-binding domain Plasmids. Currently, there are a variety of commercially available and academically available Y2H systems. DEEPN can accommodate many of these provided that the bait plasmid expressing the protein of interest fused to a DNA-binding domain is in a TRP1-containing plasmid. Other downstream requirements are that the sequence immediately upstream of the prey library insert is known and that a positive Y2H interaction can be scored by the production of His3 allowing for selection in media lacking histidine. Here we will describe use of a new Y2H bait plasmid (pTEF-GBD, Figure 2), however, other Y2H bait plasmids including pGBKT7 can be used as well. For construction and evaluation of bait plasmids, we will describe use of pTEF-GBD. As a general note, we recommend gene synthesis to produce an open-reading frame that adheres to the yeast codon bias to help ensure good expression and ease with cloning. Ensure that the cloning scheme allows for the bait to be in-frame with the Gal4 DNA-binding domain and that when cloning into the 3' site, a stop codon follows the bait-coding region.
- Prepare the plasmid vector. Plasmid pTEF-GBD allows for cloning a fragment encoding the protein of interest either 5' or 3' of the region encoding the Gal4 DNA-binding domain using a rapid assembly method. For insertion at the 5' site, digest 3 µg of pTEF-GBD with NarI and EcoRI or for insertion at the 3' site, digest with BamHI and XhoI for 2 – 4 h. Electrophorese sample in 1% DNA agarose gel containing 0.2 - 0.5 µg/mL ethidium bromide (EtBr) at 100 V. Excise the cut 5,630 bp TEF-GBD and purify using a DNA gel extraction kit in accordance to the manufacturer's instructions and quantify DNA by absorbance at 260 nm by spectrophotometer12.
NOTE: Generation of bait-encoding inserts. DNA fragments encoding proteins or protein fragments of interest can be made using gene synthesis and available as uncloned fragments. It is recommended that codons are optimized for expression in Saccharomyces cerevisiae and online tools for codon optimization are included in the list of materials. - For 5' insertion, flank the DNA fragment encoding an ATG start codon by 5'-TTAAGAAAAACAAACTGTAACGAATTC-3' and 5'-GCGCCTATGTGTGAACAAAAGCTTATT-3', respectively. For 3' insertion in frame with the Gal4 DNA binding domain, flank the encoding fragment by 5'- ctgcatatggccatggaggccgaa -3' and 5'-tagtaactagcataaccccttggggcc-3'.
- For plasmid construction, use the rapid assembly method as specified in manufacturer's directions for cloning fragments into cut pTEF-GBD.
- Plate all transformed E. coli onto LB-Kanr plates and incubate for 16 - 20 h at 37 °C. Colonies housing pTEF-GBD with the desired insert can be identified by PCR amplification using the oligonucleotides: 5'- CGGTCTTCAATTTCTCAAGTTTCAG -3' and 5'-GAGTAACGACATTCCCAGTTGTTC-3' for 5' insert and 5'-CACCGTATTTCTGCCACCTCTTCC-3' and 5'-GCAACCGCACTATTTGGAGCGCTG-3' for 3' insert. These oligonucleotides can also serve as primers for sequencing the insert.
- Plan on preparing >10 µg of each pTEF-GBD derivative and pTEF-GBD alone to provide material for sequencing and yeast transformations.
- Use the following PCR conditions: 3 min at 98 °C, followed by 25 cycles of 30 s at 98 °C, 30 s at 55 °C, and 2 min at 72 °C, followed by 5 min at 72 °C using a buffer containing 2.5 mM MgCl2, 0.5 U/100 µL DNA polymerase, and proprietary buffer.
3. Expression of Gal4-DNA-binding Domain Fusion Proteins
- Make Competent Yeast.
- Streak out PJ69-4A yeast onto a YPD plate by taking a sterile wooden applicator, scraping 1 mm3 of a -80 °C frozen stock and rubbing it gently across the YPD plate. Move the wooden applicator down the plate so that each pass goes across an untouched part of the media surface. Incubate the YPD plate at 30 °C for 2 days or until single colonies are visible. Make the frozen stock of PJ69-4A yeast by suspending yeast in water or growth media, supplementing with DMSO to 7%, and storing at -80 °C.
- Inoculate a single colony in 5 mL of culture of YPD in a 20 mm x 150 mm culture tube using a sterile wooden applicator and grow overnight at 30 °C in a shaking incubator at 200 rpm.
- Inoculate 50 mL of YPD in a 250 mL of sterile Erlenmeyer flask with 4 mL of the overnight culture of PJ69-4A yeast strain. Grow in a shaking incubator at 30 °C, 200 rpm to an optical density (OD600) of approximately 1.2, as determined by spectrophotometry with a standard 1 cm light path. Growth usually takes 5 – 7 h.
- Isolate yeast by sedimentation in a 50 mL of conical tube at 4,696 x g for 5 min at room temperature in a benchtop centrifuge. Discard supernatant by dumping into liquid waste. Using a pipette, resuspend the pellet in 5 mL of transformation buffer and transfer to 15 mL of conical tube. Resediment to discard supernatant, and resuspend the yeast in 1 mL of final volume of transformation buffer with a 1000 µL pipette.
- Incubate yeast cells for 60 min at 30 °C while shaking at 200 rpm and then place on ice for 30 – 90 min.
- Yeast plasmid transformation.
- In 1.5 mL of sterile microcentrifuge tube, add 1 µg of pTEF-GBD-based plasmid and 5 µL of 10 mg/mL Salmon sperm carrier DNA solution. Also include a tube containing only Salmon sperm carrier DNA as a negative transformation control. Add 100 µL of the ice-cold yeast cell suspension to each tube by pipette. Add 100 µL of 70% PEG solution with a 1000 µL pipette and mix gently by flicking the tube 5 – 10 times (do not vortex).
- Incubate at 30 °C, in a shaking incubator at 200 rpm for 45 min.
- Heat shock at 42 °C for 15 min.
- Sediment in a microcentrifuge at 845 x g for 3 min at room temperature, pipette off and discard supernatant, resuspend pellet in 150 µL of sterile water by pipetting up and down, and spread over the surface of an CSM-Trp plate.
- Place plates right side up in 30 °C incubator and incubate for 2-3 days until colonies are visible. Plates may be turned upside down to avoid condensation on the plate surface after incubation at 30 °C for 6 – 12 h.
- Take 2 – 3 colonies per transformation and streak as a patch onto a CSM-Trp plate using a sterile toothpick. Allow to grow for 24 h at 30 °C.
- Make lysates for protein expression.
- Inoculate 3 mL of CSM-Trp liquid media with a match-head-size of yeast from the patch and grow overnight at 30 °C in a 20 mm x 150 mm culture tube, while shaking at 200 rpm. Make two overnight cultures per bait and empty pTEF-GBD vector.
- Add 1 mL of YPD to each 3 mL of CSM-Trp overnight culture. Grow for 1 h at 30 °C, while shaking at 200 rpm. Check the OD of cells by spectrophotometer.
- Sediment an equivalent number of cells, normalizing according to the OD. Use sterile 1.5 mL of microcentrifuge tubes with a 5 min spin at 2,348 x g at room temperature in a microcentrifuge. Use a pipette to discard supernatant. The final stock corresponds to a minimum of 2.1 OD.
NOTE: When calculating equivalent number of cells, it can occur that different volumes may be required from each overnight culture to achieve the minimal 2.1 OD. - Resuspend the pellet in 450 µL of 0.2 M NaOH by pipetting up and down. Incubate for 5 min at room temperature. Recentrifuge cells for 2 min at 2,348 x g at room temperature, and discard the supernatant by pipette.
- Resuspend the pellet with 50 µL of TWIRL buffer by pipetting up and down carefully as to not make bubbles. Heat sample for 5 min at 70 °C.
- Check for protein expression by SDS-PAGE.
- Use a gradient gel of 4 – 20% to ensure a large range of molecular weights can be resolved. Load equivalent amount (same OD) of samples into an SDS-PAGE gel and be sure to include at least one sample containing the unmodified pTEF-GBD vector13,14,15.
- After electrophoretic separation, transfer gel to nitrocellulose and immunoblot using anti-myc monoclonal or polyclonal antibodies and ECL detection solution (Figure 3).
4. Self-activation Test
- Streak out the MATalpha yeast from the -80 °C stock corresponding to the strain housing the prey library of interest onto a YPD plate by taking a sterile wooden applicator, scraping a small amount of yeast out of the vial and streaking it across the YPD plate. Incubate the YPD plate at 30 °C for 2 days or until single colonies are visible. Patch a couple single colonies onto a YPD plate and incubate overnight at 30 °C.
NOTE: The new strain developed here to house prey library is PLY5725 whereas some compatible commercially available Y2H libraries are housed in Y187. - Follow the procedures in 3.3.1 – 3.3.4 to transform PLY5725 with the LEU2-based plasmid used to house the desired prey library. For libraries developed here, the corresponding plasmid is pGal4AD (pPL6343). To recover yeast transformants, plate onto CSM-Leu-Met plates. After colonies arise, streak as patches onto a CSM-Leu-Met plate and incubate for 24 h at 30 °C.
- Follow the protocol in 3.4 to confirm expression of pPL6343 empty vector using anti-HA monoclonal or polyclonal antibodies and ECL detection solution.
- Streak each of the transformed PJ69-4A yeast in a cross pattern with PLY5725 constructs on a YPD plate that was confirmed to express protein in protocol section 3.4 and 4.3 and incubate at 30 °C overnight. Take 1 mm3 of the cells where the two strains have grown together and patch onto separate CSM-Leu-Met, CSM-Trp, and CSM-Trp-Leu plates and grow 24 h.
NOTE: Desired diploids will grow on the CSM-Trp-Leu plates. Growth on CSM-Leu-Met and CSM-Trp plates serve as a positive control for yeast growth. - Grow diploids in 1 mL of CSM-Trp-Leu media overnight at 30 °C. Sediment 500 µL cells in a 1.5 mL of microcentrifuge tube at 2,348 x g, 3 min at room temperature in a microcentrifuge. Discard supernatant by pipette. Resuspend the cells in 1 mL of sterile water and repeat sedimentation and resuspension. Check the OD600 of cells.
- Make a series of 1:10 serial dilutions of each cell suspension using sterile water with the starting most concentrated solution of each at an OD of 0.5. Spot 5 µL of each dilution onto a CSM-Leu-Trp plate, a CSM-Leu-Trp-His plate, and a CSM-Leu-Trp-His+3AT plate. Incubate at 30 °C and inspect for growth daily over 3 days (Figure 4).
NOTE: For the 1:10 serial dilution, pipette 10 µL of the tube containing an OD 0.5 into 90 µL of water and mix by pipetting up and down. Continue to make 1:10 serial dilutions until there is a total of six different concentrations to spot.
5. Create Yeast Populations with Bait and Prey Library
NOTE: The Y187 strain that houses commercial prey library plasmids does not mate well. Thus, the following optimized conditions are required to maintain complexity of the library. The PLY5725 strain containing Y2H prey libraries mates better and the same mating procedure can be used with this strain (Figure 5).
- Inoculate a 3 mL of cultures of each of the PJ69-4A transformants carrying the various TRP1-containing pTEF-GBD bait plasmid in CSM-Trp media in a culture tube. Include two separate cultures containing the pTEF-GBD vector plasmid alone to serve as procedural controls. Incubate cultures at 30 °C, 200 rpm for 6 h and then dilute into a 25 mL of culture in a sterile Erlenmeyer flask for overnight growth.
- Thaw a frozen (-80 °C) vial of the MATalpha cells containing the LEU2-carrying "prey" library at room temperature. Inoculate a 125 mL of CSM-Leu-Met media in a sterile Erlenmeyer flask with the whole thawed vial. Grow all cultures overnight at 30 °C with shaking at 200 rpm.
NOTE: The OD600 of the overnight cultures needs to range between 1.0 to 1.5 before proceeding to next steps. - Centrifuge 21 OD equivalents of each of the PJ69-4A transformant cultures with a 5 min spin at 4,696 x g at room temperature. For every 10 mating reactions desired, pellet 39 OD600 equivalents of the MATalpha strain carrying the library plasmids in separate 50 mL of conical tubes.
- Resuspend cells in 10 mL of sterile water and re-pellet in new 50 mL of conical tube 4,696 x g 5 min at room temperature in a benchtop centrifuge. Using a pipette, gently remove the supernatant without disrupting the pelleted cells.
- Resuspend pellets of PJ69-4A cells in 4 mL of and PLY5725 cells in 10 mL of bYPDA (pH 3.7).
- To set-up mating reactions, add 1 mL of PJ69-4A transformed cells, 1 mL of MATalpha library-containing cells, and 1 mL of bYPDA pH 3.7 to new 50 mL of conical tube. Incubate at 30 °C with gentle orbital agitation (100 – 130 rpm) for 90 min.
- Centrifuge cells for 5 min at 4,696 x g, at room temperature in a benchtop centrifuge. Remove supernatant by pipette and resuspend the pellet in 2 mL of 1:1 bYPDA:YPD. Plate all 2 mL of onto a 100 mm YPD plate by pipette and incubate at 30 °C for approximately 20 h.
- Harvest cells from the YPD plates using a cell scraper to dislodge the cells into 2 – 3 mL of CSM-Leu-Trp media. Pipette dislodged cells into a 50 mL of conical tube. Rinse the plates 4 – 5 times with 2 – 3 mL of CSM-Leu-Trp media by pipetting up the media using a 1000 µL pipette and gently ejecting the media across the YPD plate surface.
- Centrifuge cells for 5 min at 4,696 x g at room temperature in a benchtop centrifuge. Discard supernatant by pipette and resuspend the cells in 40 mL of CSM-Leu-Trp media by pipetting up and down (do not vortex).
- To estimate the number of diploid cells formed, dilute 4 µL of the diploid mixture into 200 µL and 2000 µL CSM-Trp-Leu media. Plate 200 µL of each dilution onto a CSM-Leu-Trp plate.
NOTE: The two plates represent a 1:10,000 and 1:100,000 fold dilution of the stock of diploids harvested and yielding an expected ~9,000 – 27,000 colonies on the 1:10,000 dilution plate after incubation at 30 °C for 36 – 40 h. Check plates after step 5.7. A minimum number of 200 colonies on the 1:10,000 dilution plate is required to proceed to step 5.8 - Immediately take the remainder of each 40 mL of cell resuspension and inoculate a 1,000 mL of Erlenmeyer flask containing 500 mL of CSM-Leu-Trp media. Take an initial OD600. Incubate these flasks at 30 °C with shaking at 180 rpm until they reach saturation (~2.0 OD/mL). This typically takes about 36 – 40 h. Monitor growth at 24 h then again at 36 h by OD600.
- Using a pipette, remove 20 mL of aliquots from each of the saturated 500 mL of cultures and innoculate 2,000 mL of Erlenmeyer flasks, one containing 750 mL of CSM-Leu-Trp media and the second containing 750 mL of CSM-Leu-Trp-His with the lowest level of 3AT that eliminates background (previously determined in Section 4.5). Mix the new cultures (770 mL) well by swirling and take an initial OD600.
- Incubate cultures at 30 °C while shaking at 180 rpm until reaching saturation, which typically occurs within 24 h for the unselected CSM-Leu-Trp culture and can take over 70 h for cultures under selection for Y2H interactions.
- Once cultures have reached saturation (OD ~2.0), remove 11 mL of by pipette, sediment the cells with a 5 min spin at 4,696 x g at room temperature, discard supernatant by pipette, and freeze at -20 °C or continue onto DNA extraction. The selected and unselected samples will both be used for deep sequencing.
6. Sample preparation for DEEPN Deep Sequencing
- DNA extraction.
- Use a pipette to resuspend cell pellets from protocol section 5.7 in 500 µL of sTE buffer and transfer to a 1.5 mL of microcentrifuge tube. Add 3 µL of betamercaptoethanol and 10 µL of Zymolase stock. Mix well and incubate in the 37 °C incubator for 24-36 h.
- Extract the sample two times with 500 µL phenol/chloroform/isoamyl alcohol while using a fume hood16.
- Add 7 µL of 4 M NaCl, 900 µL of ice cold 100% ethanol (ETOH), mix by inversion and either freeze -20 °C or continue to sediment DNA by spinning at 21,130 x g for 10 min at room temperature in a microcentrifuge.
- Discard supernatant by pipette. Wash the pellet thrice with 900 µL of 70% ETOH.
- Sediment pellet 21,130 x g for 2 min and remove residual ETOH wash by pipette. Dry pellet for 7 min at 42 °C.
- Resuspend pellet in 120 µL of 0.1x sTE in a 37 °C water bath for 90 min, flick the tubes to mix every 30 min.
- Pipette 60 µL of extracted DNA into a sterile 1.5 mL of microcentrifuge tube. Add 120 µL of sTE, 3.5 µL of RNase A stock, flick to mix and incubate at 37 °C for 1 h.
- Ethanol precipitate as previously done in Section 6.1.3 – 6.1.5, but use 7 µL of 5 M ammonium acetate instead of 4 M NaCl.
- Resuspend RNase A-treated DNA in 55 µL of 0.1x sTE in a 37 °C water bath for 90 min, flick the tubes to mix every 30 min. Quantify DNA by absorbance at 260 nm on a spectrophotometer.
- PCR cDNA inserts.
- Perform two, 50 µL PCR reactions per DNA sample. Each reaction contains 25 pmol of each forward and reverse primer matching the prey-library plasmid (see materials). Reactions also contain 25 µL of High-Fidelity 2x PCR Master Mix, 5 µg of DNA sample, and water up to 50 µL. Amplify reactions for 25 cycles with extension times of 3 min at 72 °C, an anneal temperature of 55 °C for 30 s, and denaturing at 98 °C for 10 s. Precede cycling by a 30 s denaturation at 98 °C and follow with a 5 min incubation at 72 °C.
- Analyze 4 µL of each PCR reaction by 1% DNA agarose gel electrophoresis with the DNA agarose gel containing 0.2 – 0.5 µg/mL EtBr17. Visualize DNA sample by UV transillumination. Samples will show a smear of DNA around 1 – 3 kb, where the banding pattern may be found for samples where a Y2H interaction was selected (Figure 6).
- Combine duplicate PCR samples and purify using the PCR purification kit in accordance to the manufacturer's instructions and quantify DNA by absorbance at 260 nm on a spectrophotometer.
7. Deep sequencing
NOTE: Sample preparation and sequencing on a deep sequencing platform is typically available in commercial and academic DNA sequencing core facilities.
- Shear 600 ng of PCR product using a high performance ultra-sonicator to give fragments of an average length of ~300 bp.
- Generate indexed sequencing libraries using a preparation kit for deep sequencing that adds linkers encoding barcodes, priming sites, and capture sequences asymmetrically on the ends of the DNA fragments.
- Perform library preparation according to manufacturer's instructions. Pool indexed libraries and sequence as long paired-end reads on a deep sequencing platform (e.g., 2 x 150 bp PE reads). The desired number of reads targeted for each sample is between 10 and 40 million, with more reads desired for the unselected populations that are typically more complex. We recommend at least 20 million or more reads for the unselected populations.
8. Bioinformatic Processing and Verification
- Process DNA sequencing data in the form of fastq with a set of stand-alone software programs built to (1) map sequence read files in a universal SAM format (2) quantify gene enrichment between datasets (3) perform statistical analysis of data in order to rank which candidate genes are positive for Y2H interaction (4) provide information as to what region(s) and what translational frame each of the prey cDNA per gene fragments that yield positive Y2H interactions are comprised, and (5) provide tools to reconstruct not only the 5' but also the 3' end of the interacting fragments allowing their reconstruction and verification in a traditional Y2H format. Operation of these programs is detailed in the accompanying study.
Representative Results
The Y2H assay has been widely used for finding protein:protein interactions and several adaptations and systems have been developed. For the most part, the same considerations that help ensure success with these previous approaches are important for DEEPN. Some of the important benchmarks include: ensuring expression of DNA-binding domain fusion proteins, ensuring a low background of spurious His+ growth in the diploids containing the bait of interest with an empty prey plasmid, a high mating efficiency of the bait containing MATA yeast with the library-containing MATalpha yeast, and finally, low-stringency conditions that allow the population to grow under conditions that select for many positive Y2H reactions, even ones that produce low levels of His3 activity and a weak Y2H transcriptional response.
One of the critical aspects of DEEPN is to reproducibly introduce the Y2H library into strains that contain different bait plasmids and to reliably select those populations for those library plasmids that create positive Y2H interactions. This becomes more difficult when the complexity of the Y2H library increases since it is harder to ensure adequate transfer of the entire library across different initial populations. In addition, the size of populations chosen to grow under selection conditions and depth of sequencing have to be large enough to observe reproducible changes in the Y2H plasmid composition to reliably identify true positive Y2H interactions. In previous studies, we used commercially available Y2H cDNA libraries. We found the variability in the Y2H library distribution between separate populations carrying the same bait plasmids is low, with an overdispersion between the two initial populations before selection of <0.01 typically, and 0.35 – 0.55 for separate populations after selection4. However, the complexity of some of these commercially available Y2H libraries is fairly low (Figure 7). In addition, many of the clones within it (~60%) are made entirely of cDNA fragments that are 3' of the coding region, which further limits their utility. To demonstrate that the methods above were capable of accommodating more complex Y2H libraries, we created a new Y2H library in the streamlined 'prey' plasmid vector (pGal4AD) containing fragments of genomic DNA from Saccaromyces cerevisiae (strain PLY5725). Genomic DNA was fragmented by shearing, size selected for ranges of 600 – 1500 bp, modified with adaptors, and inserted into pGal4AD to create the SacCer_TAB Y2H library (Figure 8). The library was transformed into PLY5725, which produced a yeast population that was then mated to separate samples of PJ69-4A MATA carrying the pTEF-GBD plasmid alone. Duplicate diploid populations were grown under non-selective and selective conditions. The Y2H plasmid library inserts were analyzed by deep sequencing. When mapped to genomic DNA encompassing 100 bp upstream and downstream of each protein coding region (84% of the whole genome), we found >1.1 million different plasmids in the library for an estimated total size of the library in yeast of ~1.35 million different plasmids. As a comparison, we also transformed a previously described yeast genomic Y2H library11 (PJ_C1,C2,C3 Y2H library) in MATalpha yeast, and subjected it to the same analysis as above. We found that the complexity of our random fragment yeast Y2H library was far higher and had far more plasmids encoding in-frame fragments of each gene than the previously published library (Figure 9). Importantly, the generation of initial library containing diploid populations was very reproducible with an overdispersion of <0.01 (Figure 10). Moreover, the reproducibility of having the two separate yeast populations produce similar redistributions of plasmids after selecting for positive Y2H interactions was very good, yielding an overdispersion of 0.3. Thus, the methods here accommodate larger Y2H libraries of higher complexity than those previously used.
In terms of increasing the ease of DEEPN, we prefer using gene synthesis to make inserts coding for the bait protein of interest. This allows coding regions for protein domains, chimeras, and mutants to be easily incorporated into Y2H bait plasmids, but also allows their codons to be optimized for expression in S. cerevisiae. Expression of two different Gal4-DNA-binding domain fusion proteins is demonstrated in Figure 3. Two different yeast transformants expressing either of two bait fusion proteins were prepared and subjected to SDS-PAGE and immunoblotting with anti-myc antibodies. Note expression levels of bait proteins relative to the Gal4 DNA-binding domain alone from the empty vector. We found it is important not only to verify the expression of the bait fusion protein, but also to verify expression in the exact MATA yeast transformant colony that will be used to expand and mate to the prey library-containing yeast. Once a transformant has been identified, it is possible to freeze it down and store for later use.
Figure 4 shows a test for self-activation where a serial dilution of diploid cells are plated onto CSM-Leu-Trp (+His), CSM-Trp-Leu-His (-His), and CSM-Trp-Leu-His+3AT (-His+3AT) plates and allowed to grow at 30 °C for 3 days. The desired result is to observe growth in the presence but not absence of histidine regardless of whether there is 3AT. This will allow the use of CSM-Trp-Leu-His to select for yeast with a positive yeast 2-hybrid interaction. If there is growth on CSM-Leu-Trp-His plates, then a Y2H selection can still be obtained using the lowest concentration of 3AT that prevents growth. We find that if growth can be blocked in CSM-Trp-Leu-His + 0.1 mM 3AT, then the DEEPN assay can proceed using this condition to select for Y2H interactions. If, however, inhibition of the growth of diploids containing pGal4AD or other empty prey plasmid and the pTEF-GBD-bait fusion plasmid requires higher concentrations of 3AT, it will compromise the performance of the DEEPN procedure and a different bait plasmid should be sought. Note that His+ growth is the only selection for a positive Y2H interaction. We have found this is sufficient to enrich for Y2H interactions in batch.
One of the most critical procedures in the DEEPN workflow is to achieve high efficiency mating of the MATA yeast carrying the bait plasmid with the MATalpha yeast carrying the Y2H prey library. We found that some strains (e.g., Y187)18, housing commercially available cDNA libraries, have relatively poor mating efficiency. For that reason, we engineered a strain to carry Y2H libraries. This strain is based on BY4742, a derivative of S288c. This strain lacks GAL4, GAL80, TRP1, LEU2, and HIS3. It contains no reporters that are responsive to a hybrid Gal4 protein nor a different hybrid (e.g., LexA-VP16). Instead, the source of Y2H-induced His3 production is housed within the MATA strain that would carry the particular bait plasmid. This simplifies the system and allows greater flexibility in that one can use the same library-containing MATalpha cells to mate with a strain expressing a LexA-bait fusion protein and a LexA(UAS)-HIS3 reporter or a Gal4-DBD-bait fusion protein and a Gal4(UAS)-HIS3 reporter.
Once the diploid populations carrying both a bait plasmid and the library are generated, they are diluted and allowed to grow under conditions that just select for both plasmids (e.g., CSM-Trp-Leu) or for both plasmids and a positive Y2H interaction that drives production of His3 (e.g., CSM-Leu-Trp-His). It is important to start with a large amount of the starting population to avoid an evolutionary 'bottleneck' that can skew the population that results after selection for a positive Y2H interaction. Thus, our procedure specifies using 20 mL of out of the 500 mL of diploid population culture to be grown in 750 mL of fresh CSM-Leu-Trp-His media to avoid this problem. With the pTEF-GBD as the bait plasmid, we found that a single round of dilution and growth was sufficient to evolve an informative population. Using different bait plasmids previously, we used two rounds of dilution and growth, an initial 20 mL of into 750 mL, and then 2 mL of from that saturated culture diluted in 75 mL. Figure 6 shows the results from PCR amplification across the library inserts for the diploid population grown under non-selective conditions, as well as a first and second successive round of dilution and growth in selective condition for two different bait plasmids. Note that with no selection, there is a generalized smear of PCR products indicative of a complex and relatively well-normalized mixture of fragments. Upon selection, that pattern changes, with some species greatly enriching so that individual bands can be discerned. Some degree of banding is typical of the successful experiments conducted so far, yet a smear pattern in addition to, or instead of a banding pattern, is indicative of a complex mixture of prey inserts and is desirable if one wants to maximize the number of candidates that DEEPN detects. Very strong banding pattern, where most of the PCR product is found in 1 – 3 bands, indicates that most of sequencing data will be dominated by only 1 – 3 prey inserts. One can compensate for this partly by dedicating more reads from this sample. One can see that if the population is diluted and grown further (see 'selected d2' in Figure 5), the banding pattern is more prominent, indicating that prey plasmids conferring weak but authentic Y2H interactions are being diminished while a select few prey plasmids are increasing their abundance. Successive dilution and growth to this excessive level is deemed counterproductive to the goal of catching the largest number of potential candidates and thus with the protocol here, we recommend one round of dilution and growth be used.
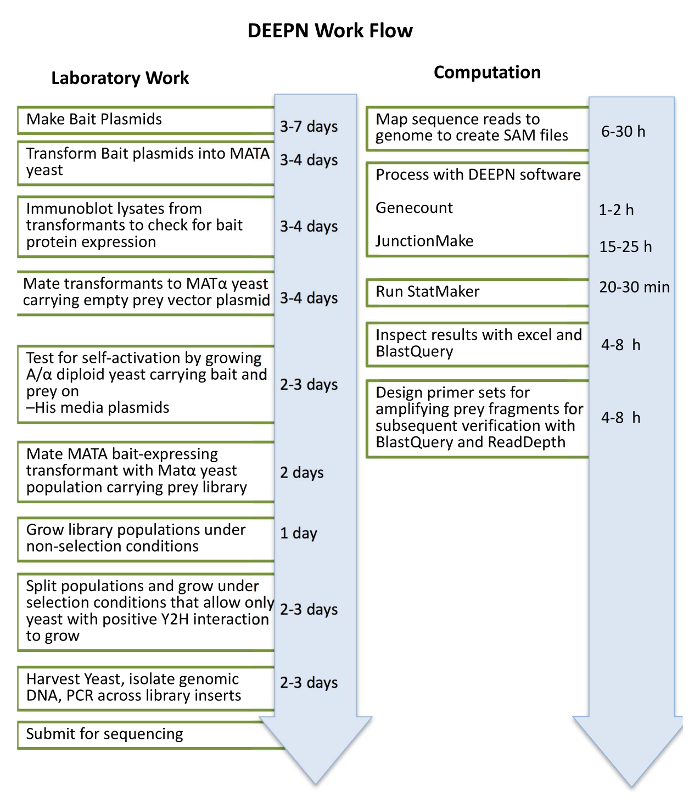
Figure 1: Schematic of DEEPN workflow. The general outline of the laboratory procedures is shown on the left along with the approximate time needed to complete the corresponding task. On the right is the bioinformatics workflow using the DEEPN and Stat_Maker software packages. Please click here to view a larger version of this figure.
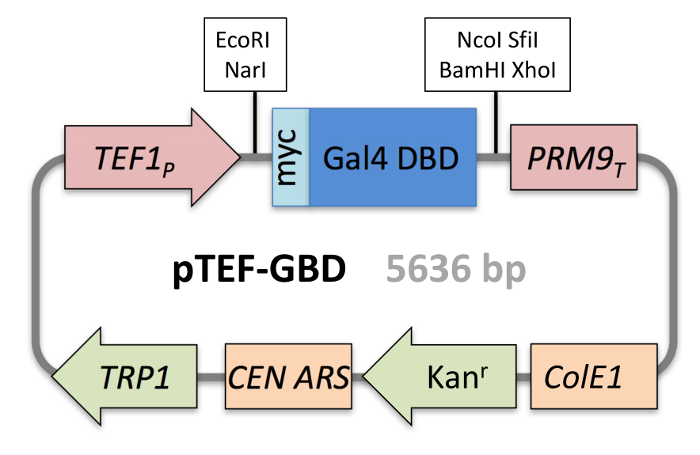
Figure 2: Schematic of pTEF-GBD. The TRP1-containing low copy Gal4 DNA-binding domain fusion protein expression plasmid is shown. It features a constitutive TEF1 promoter, myc epitope tag, the Gal4 DNA binding domain followed a T7 RNA polymerase binding site, polylinker, and PRM9 terminator within a centromere (CEN)-based plasmid. This plasmid also carries Kanamycin resistance and the ColE1 bacterial origin of replication. Please click here to view a larger version of this figure.
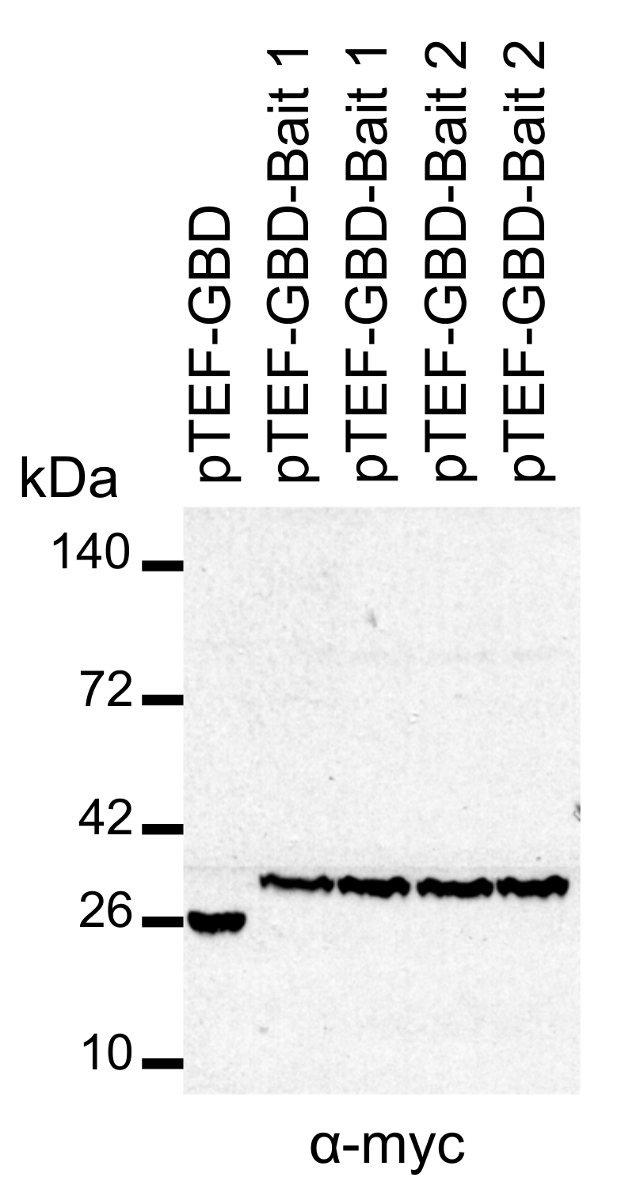
Figure 3: Expression of Gal4-bait fusion proteins. Lysates from yeast expressing different bait proteins fused to the Gal4 DNA-binding domain expressed in pTEF-GBD were subjected to SDS-PAGE and immunoblotting with anti-myc antibodies. pTEF-GBD expresses just the Gal4-DNA-bindng domain alone; pTEF-GBD-bait1 expresses the Gal4-DNA-bindng domain fused to a RhoA lacking its C-terminal prenylation site and housing a mutation locking it into a GTP-bound conformation; pTEF-GBD-bait2 expresses the Gal4-DNA-bindng domain fused to a RhoA lacking its C-terminal prenylation site and housing a mutation locking it into a GDP-bound conformation. Please click here to view a larger version of this figure.
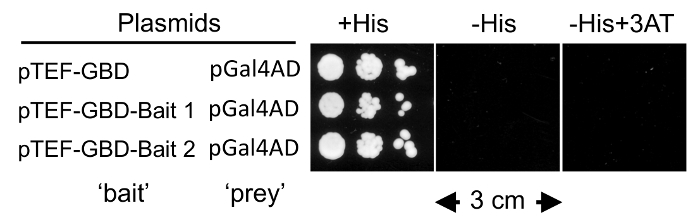
Figure 4: Self-Activation Test. Diploids made from PJ69-4A cells carrying the indicated bait plasmids and PLY5725 cells carrying the indicated prey plasmid were serially diluted and spotted onto CSM-Leu-Trp plates, CSM-Leu-Trp-His plates, and CSM-Leu-Trp-His+3AT plates and grown for 3 days at 30 °C. The PJ69-4A transformants used for mating were the first of each pair shown in Figure 3. Please click here to view a larger version of this figure.
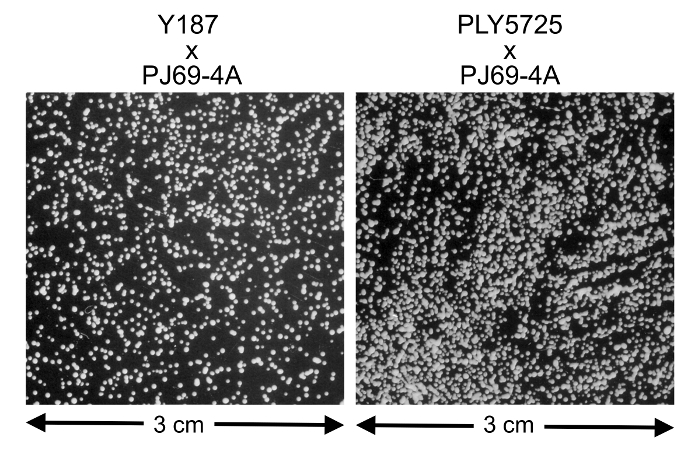
Figure 5: Mating efficiency of Y187 vs. PLY5725. The mating reaction between PJ69-4A containing a TRP1-containing bait vector and Y187 or PLY5725 carrying prey plasmid was performed. 1:10,000 dilutions were plated onto CSM-Trp-Leu to select for diploids. The PLY5725 strain shows a higher mating efficiency than the Y187 strain with ~10 fold more colonies produced. Please click here to view a larger version of this figure.
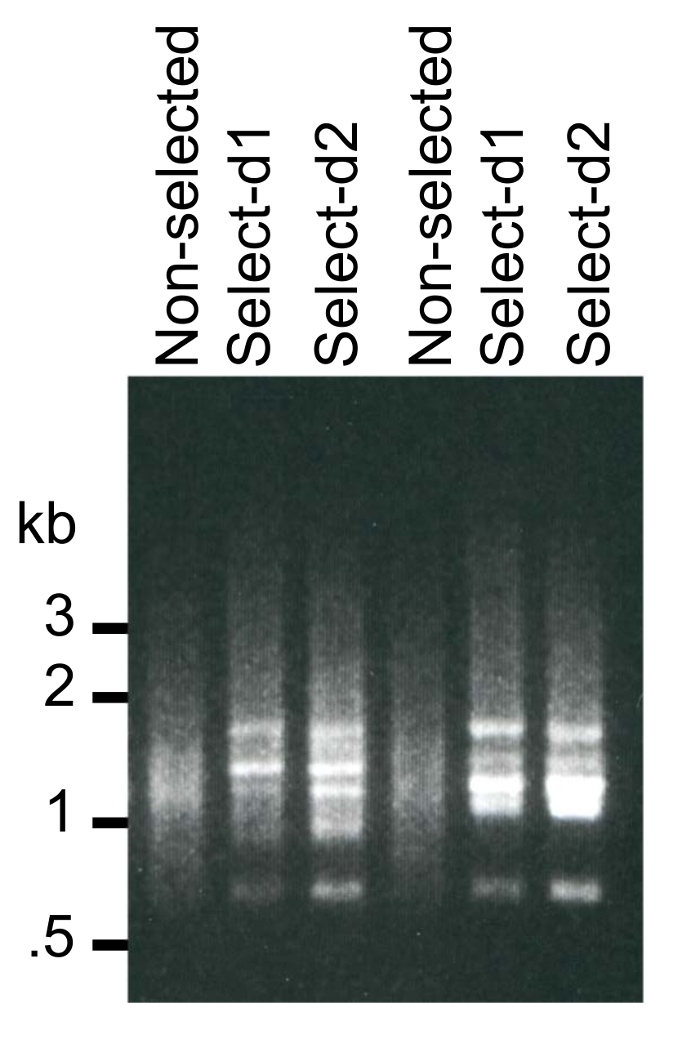
Figure 6: PCR of prey library inserts. DNA was isolated from diploid yeast containing a prey library that was grown under non-selective conditions (CSM-Leu-Trp media) or conditions selecting for a positive Y2H interaction (CSM-Leu-Trp-His media). PCR across the library inserts reveals differences in the repertoire of inserts selected. Two rounds of selective growth were used. An initial round of growth made by diluting 20 mL of the 500 mL of diploid culture into 750 mL of non-selective CSM-Leu-Trp media, and another 20 mL of into 750 mL of CSM-Leu-Trp-His media for an initial round of selective growth (d1). This was followed by an additional round of growth (d2) made by taking 2 mL of the d1 culture and diluting into 75 mL of selective media CSM-Leu-Trp-His. Please click here to view a larger version of this figure.
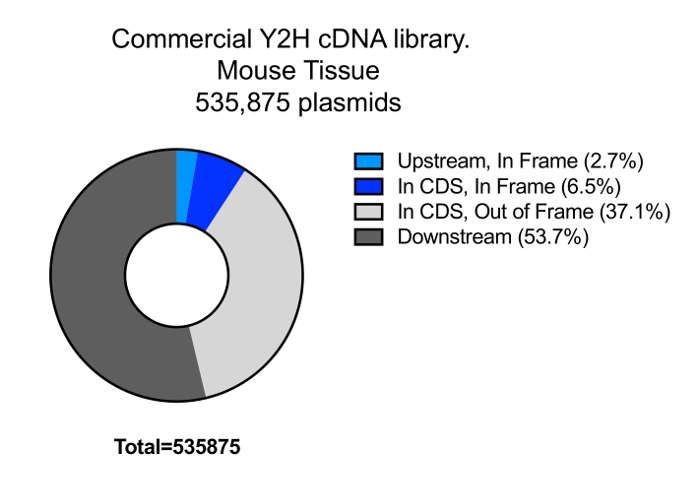
Figure 7: Complexity of Y2H cDNA library. Analysis of the content of a commercially available mouse cDNA Y2H prey libraries: a library from cDNA of mouse brain and one from multiple mouse tissues. Please click here to view a larger version of this figure.
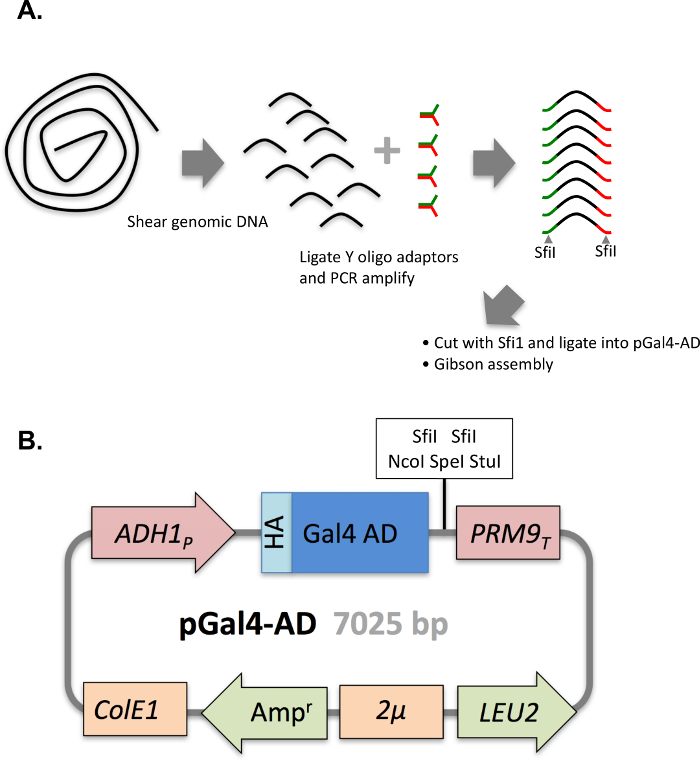
Figure 8: Generation of Y2H yeast genomic fragment library. A. Schematic describing assembly of the yeast genomic fragment library into pGal4AD. Genomic DNA from strain PLY5725 was randomly sheared, ligated with the indicated Y-adaptors, and ligated into SfiI-cut pGal4AD. Ligations were transformed into bacteria to yield 2.2 x 106 independent colonies that were combined and grown prior to isolation of their plasmid DNA to comprise the SacCer_TAB Y2H library. B. The LEU2-containing plasmid (pGal4AD) housing the Gal4 transcriptional activation domain is shown. Please click here to view a larger version of this figure.
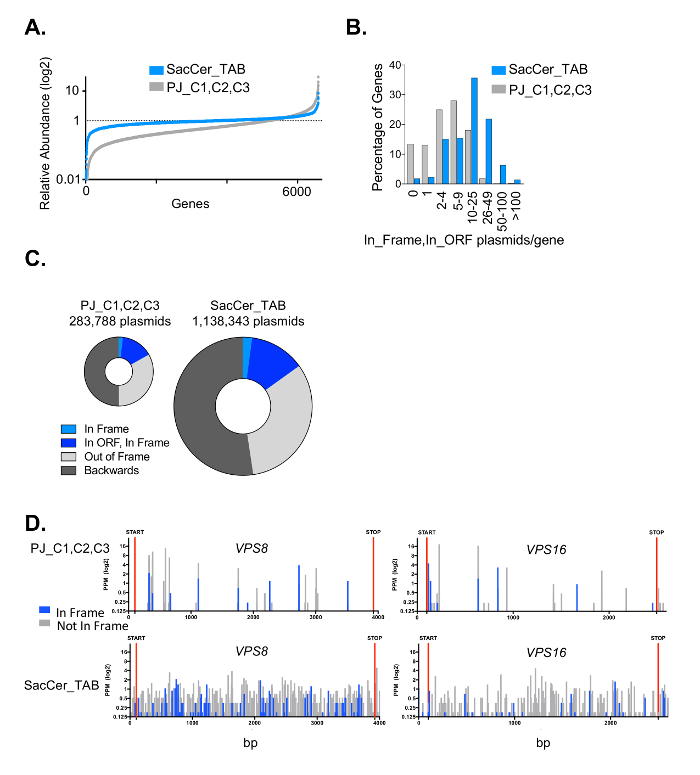
Figure 9: Complexity of Y2H yeast genomic library. The SacCer_TAB genomic library was transformed into the PLY5725 MATalpha strain and the PJ_C1,C2,C3 yeast genomic library was transformed into the Υ187 ΜΑΤalpha strain. Diploids of these populations were made by mating to the PJY69-4A strain. Populations were grown for 10 generations and prey fragments PCR amplified were subjected to high-throughput sequencing and analysis. A. Shows the rank order of reads per each gene in the Y2H libraries divided by the reads per gene found by sequencing the yeast genome. Given equivalent abundance of each gene, each gene would have a value of 1. B. Histogram showing the number of unique plasmids per gene that encode a fragment that is both in the protein coding region (ORF) and in the proper translational reading frame. C. Comparison of the number of different plasmids in each library that encode yeast genes and the proportion of these that are and are not in the correct translational reading frame or are inserted backwards. D. Plots showing positions and abundance of the junctions that map to example genes (VPS8 and VPS16) found for the plasmids in each library. Plasmids with gene fusions that are in the correct translational reading frame are designated blue. Please click here to view a larger version of this figure.
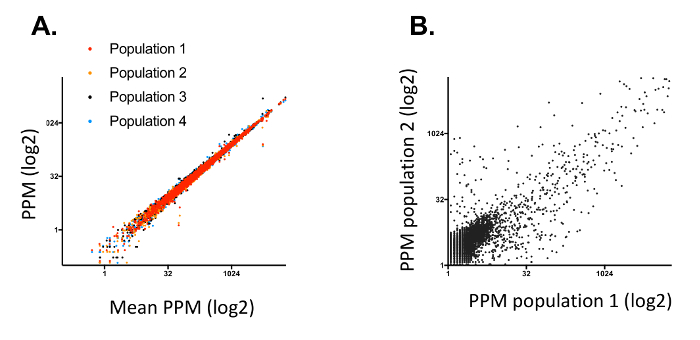
Figure 10: Reproducibility of DEEPN with complex Y2H yeast genomic library. A. Four different yeast diploid populations were created by mating with the SacCer_TAB Y2H library housed in PLY5725, grown under non-selective conditions, and sequenced. The reads per gene for each population individually is plotted as a function of the average rank order value across all populations. B. Shows the reads per gene between two samples after selection for positive Y2H interactions. Please click here to view a larger version of this figure.
Discussion
Here we provide a guide for how to perform Y2H assays in batch using optimized methods. There are a few critical steps in the procedure to help ensure that the population of yeast that would be placed under selection is representative of the starting library and that enough of the starting yeast population is used to undergo selection to limit variability. Importantly, these benchmarks are relatively easy to achieve alongside adapting the methods and materials for a traditional Y2H assay, thus making this approach accessible to most laboratories equipped for standard molecular biology. DEEPN allows selection from the same prey library population while using different bait plasmids. Hence, the set of interacting candidates that produce a Y2H interaction with one bait vs. another can be directly compared. Because deep sequencing is used, one can verify the starting library composition for each bait-specific yeast population and follow the enrichment of each candidate prey gene in the library independently. Batch processing allows querying the same library against different baits in a semi-quantitative manner that then allows one to calculate a statistical ranking4.
There are several steps in this protocol that are critical for success. One is that a large number of diploids must be obtained to ensure adequate representation of the Y2H prey library is mixed with each bait plasmid of interest. The mating procedure described here has been optimized by varying several parameters. We found that some strains that house commercial Y2H libraries mate poorly in general, however, the mating procedure described here can generate 2 x 106– 2 x 107 diploids when followed, allowing adequate and reproducible transfer of the Y2H library into the population. Another critical aspect of the procedure is to ensure that the bait plasmid of interest does not create a positive Y2H interaction on its own or with the empty Gal4 activation domain prey plasmid. The method for selecting a Y2H interaction is demanding growth in the absence of Histidine wherein a positive Y2H interaction induces transcription of HIS3 to allow cells to be His+. While routinely the traditional Y2H assays can add the competitive inhibitor 3AT to diminish background growth or use other reporters3 , this procedure works best when cells with weak Y2H interactions can grow and thus increasing the stringency for growth and selecting only for cells with strong Y2H interactions limits the repertoire of prey plasmids that can be identified. Another critical aspect is to make sure that a large amount of the starting diploid population used to grow under selective and non-selective conditions to avoid evolutionary bottlenecks from sampling error4 . Following the culture volumes and cell numbers specified here will help ensure reproducibility and diminish noise.
One of the reasons the DEEPN approach is powerful is that it can comprehensively follow all the plasmids in a given prey library for their ability to interact with the bait of interest. Thus, one of the limitations of DEEPN is the complexity of the library used. For some of the commercial libraries like the ones we used here, we found that the number of plasmids that encode bona fide fragments of cDNA ORF that are in the same reading frame of Gal4 activation domain is between 3 – 6 x 104 with representation of ~6,000 – 8,000 different genes. We found that nearly 75% of these libraries contained fragments corresponding solely to cDNA regions that were 3' of the coding DNA sequence (CDS/ORF). Moreover, almost a third of the genes which had fragments in the library had none that corresponded to regions in the ORF or upstream of the ORF.
We made three other changes to the DEEPN method. One is a new MATalpha strain that can carry 'prey' libraries housed within a TRP1 plasmid and that mates far better than some commercially available library-containing strains such as Y187. This helps ensure complete transfer of the library population for each bait of interest. We also made a new 'bait' expression plasmid, which differs from previously described plasmids that use a variable copy 2µ-based backbone10. Here we describe a low-copy CEN plasmid (pTEF-GBD) and find that a single round of selective growth is sufficient to enrich for interacting prey plasmids. Lastly, we build a streamlined 'prey' library vector and used to produce a high-density random-fragment genomic Y2H library for Saccharomces cerevisiae, which should be helpful in the future for discovering and characterizing interactions amonst yeast proteins. Overall, the improvements in materials and bioinformatics tools (described in the accompanying work) make the DEEPN approach an accessible, feasible, and efficient way of performing comprehensive and comparative Y2H screens.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank the staff within the Institute of Human Genetics for NGS library preparation and sequencing. We thank Einat Snir for her expertise in preparing genomic library fragments for the Y2H plasmid library made here. This work was supported by National Institutes of Health: NIH R21 EB021870-01A1 and by NSF Research Project Grant: 1517110.
Materials
| Illumina HiSeq 4000 | Illumina | deep sequencing platform | |
| Monoclonal anti-HA antibodies | Biolegend | 901514 | Primary Antibody to detect expression of HA in pGal4AD constructs |
| Polyclonal anti-myc antibodies | QED Biosciences Inc | 18826 | Primary Antibody to detect expression of MYC in pTEF-GBD constructs |
| NarI | New England BioLabs | R0191S | |
| EcoRI-HF | New England BioLabs | R3101S | |
| BamHI-HF | New England BioLabs | R3236S | |
| XhoI | New England BioLabs | R0146S | |
| Polyethylene Glycol 3350, powder | J.T. Baker | U2211-08 | |
| Salmon Sperm DNA | Trevigen, Inc sold by Fisher Scientific | 50-948-286 | carrier DNA for yeast transformation section 3.2.1. |
| Kanamycin Monosulfate | Research Products International | K22000 | |
| LE Agarose | GeneMate | E-3120-500 | used for making DNA agarose gels |
| Sodium Chloride | Research Products International | S23025 | |
| Tryptone | Research Products International | T60060 | |
| D-Sorbitol | Research Products International | S23080 | |
| Lithium Acetate Dihydrate | MP Biomedicals | 155256 | |
| Calcium Chloride | ThermoFisher | C79 | |
| EDTA Sodium Salt | Research Products International | E57020 | |
| Yeast Extract Powder | Research Products International | Y20020 | |
| Yeast Nitrogen Base (ammonium sulfate) w/o amino acids | Research Products International | Y20040 | |
| CSM-Trp-Leu+40ADE | Formedium | DCS0789 | |
| CSM-Trp-Leu-His+40ADE | Formedium | DCS1169 | |
| CSM-Leu-Met | Formedium | DCS0549 | |
| CSM-Trp-Met | Bio 101, Inc | 4520-922 | |
| L-Methionine | Formedium | DOC0168 | |
| Adenine | Research Products International | A11500 | |
| D-(+)-Glucose | Research Products International | G32045 | |
| Bacto Agar | BD | 214010 | used for making media plates in section 1 |
| Peptone | Research Products International | P20240 | |
| 3-amino-1,2,4 Triazole | Sigma | A8056 | |
| 2-Mercaptoehanol (BME) | Sigma-Aldrich | M6250 | |
| Zymolyase 100T | USBiological | Z1004 | |
| Potassium phosphate dibasic | Sigma | P8281 | |
| Phenol:Chloroform:IAA | Ambion | AM9732 | |
| Ammonium Acetate | Sigma-Aldrich | 238074 | |
| Ethanol | Decon Laboratories, INC | 2716 | |
| RNAse A | ThermoFisher | EN0531 | |
| Urea | Research Products International | U20200 | |
| SDS | Research Products International | L22010 | |
| glycerol | Sigma Aldrich | G5516 | |
| Tris-HCl | Gibco | 15506-017 | |
| bromophenol blue | Amresco | 449 | |
| Gibson Assembly Cloning Kit | New England Biolabs | E5510S | Rapid assembly method for cloning of plasmids in section 2 |
| NEBNext High-Fidelity 2x PCR Master Mix | New England Biolabs | M0541S | Used for amplification of products for Gibson Assembly in Section 2.3 as well assample preparation for DEEPN deep sequencing in section 6.2.1 |
| Ethidium Bromide | Amresco | 0492-5G | |
| QIAquick PCR purification kit | Qiagen | 28104 | Used for purification of pcr products in section 6.2.3 |
| Qiaquick DNA Gel Extraction Kit | Qiagen | 28704 | Used for purification of digested pTEF-GBD in section 2.1 |
| KAPA Hyper Prep kit | KAPA Biosystems | KK8500 | preparation kit for deep sequencing |
| Codon optimization | http://www.jcat.de | ||
| Codon optimization | https://www.idtdna.com/CodonOpt | ||
| gBlocks | Integrated DNA Technologies | DNA fragments used for cloning in Section 2.2 | |
| Strings | Thermofisher | DNA fragments used for cloning in Section 2.2 | |
| GenCatch Plasmid DNA mini-prep Kit | EPOCH Life Sciences | Used to prepare quantities of DNA in Section 2.3 | |
| Covaris E220 | Covaris | high performance ultra-sonicator in section 7 | |
| oligo nucelotide 5’- CGGTCTT CAATTTCTCAAGTTTCAG -3’ |
Integrated DNA Technologies or Thermofisher | used for pcr amplification and sequencing 5' insert pTEF-GBD during plasmid construction | |
| oligo nucelotide 5’-GAGTAACG ACATTCCCAGTTGTTC-3’ |
Integrated DNA Technologies or Thermofisher | used for pcr amplification and sequencing 5' insert pTEF-GBD during plasmid construction | |
| oligo nucelotide 5’-CACCGTAT TTCTGCCACCTCTTCC-3’ |
Integrated DNA Technologies or Thermofisher | used for pcr amplification and sequencing 3' insert pTEF-GBD during plasmid construction | |
| oligo nucelotide 5’-GCAACCGC ACTATTTGGAGCGCTG-3’ |
Integrated DNA Technologies or Thermofisher | used for pcr amplification and sequencing 3' insert pTEF-GBD during plasmid construction | |
| oligonucleotide 5’-GTTCCGATG CCTCTGCGAGTG-3’ |
Integrated DNA Technologies or Thermofisher | 5' Pimer used for insert amplification of pGAL4AD | |
| oligonucelotide 5’-GCACATGCT AGCGTCAAATACC-3’ |
Integrated DNA Technologies or Thermofisher | 3' Pimer used for insert amplification of pGAL4AD | |
| oligonucelotide 5’-ACCCAAGCA GTGGTATCAACG-3’ |
Integrated DNA Technologies or Thermofisher | 5' Pimer used for insert amplification of pGADT7 | |
| oligonucelotide 5’- TATTTAGA AGTGTCAACAACGTA -3’ |
Integrated DNA Technologies or Thermofisher | 3' Pimer used for insert amplification of pGADT7 | |
| PJ69-4A MatA yeast strain | http://depts.washington.edu/yeastrc/ James P, Halladay J, Craig EA: Genomic Libraries and a host strain designed for highly efficient two-hybrid selection in yeast. GENETICS 1996 144:1425-1436 | MATA leu2-3,112 ura3-52 trp1-901 his3-200 gal4D, gal80D, GAL-ADE2 lys2::GAL1-HIS3 met2::GAL7 | |
| pTEF-GBD | Dr. Robert Piper Lab | Gal4-DNA binding doimain expression plasmid | |
| PLY5725 MATalpha yeast strain | Dr. Robert Piper Lab | MATalpha his3∆1 leu2∆0 lys2∆0 ura3∆0 gal4∆ trp1∆ Gal80∆ | |
| pGal4AD (pPL6343) | Dr. Robert Piper Lab | Gal4-activation domain expression plasmid | |
| 100 mm petri dishes | Kord-Vallmark sold by VWR | 2900 | |
| 125 mL PYREX Erlenmeyer flask | Fisher Scientific | S63270 | |
| 250 mL PYREX Erlenmeyer flask | Fisher Scientific | S63271 | |
| 1,000 mL PYREX Erlenmeyer flask | Fisher Scientific | S63274 | |
| 2,000 mL PYREX Erlenmeyer flask | Fisher Scientific | S63275 | |
| 20 X 150 mm Disposable Culture Tube | Thermofisher | 14-961-33 | |
| pipet-aid | Drummond | 4-000-100 | |
| 5 mL Serological Pipette | Denville | P7127 | |
| 10 mL Serological Pipette | Denville | P7128 | |
| 25 mL Serological Pipette | Denville | P7129 | |
| 1,000 mL PYREX Griffin Beaker | Fisher Scientific | 02-540P | |
| 1,000 mL PYREX Reuasable Media Storage Bottle | Fisher Scientific | 06-414-1D | |
| 1,000 mL graduated cylinder | Fisher Scientific | 08-572-6G | |
| SpectraMax 190 | Molecular Devices | used to measure the Optical Density of cells | |
| NanoDrop 2000 | Thermo Scientific | ND-2000 | Spectrophotometer used to quantify amount of DNA |
| Electronic UV transilluminator | Ultra Lum | MEB 20 | used to visualize DNA in an Ethidium Bromide agarose gel |
| P1000 Gilson PIPETMAN | Fisher Scientific | F123602G | |
| P200 Gilson PIPETMAN | Fisher Scientific | F123601G | |
| P20 Gilson PIPETMAN | Fisher Scientific | F123600G | |
| P10 Gilson PIPETMAN | Fisher Scientific | F144802G | |
| 1250 µL Low Retention Pipette Tips | GeneMate | P-1236-1250 | |
| 200 µLLow Retention Pipette Tips | VWR | 10017-044 | |
| 10 µL XL Low Retention Pipette Tips | VWR | 10017-042 | |
| 50 mL conical tube | VWR | 490001-627 | |
| 15 mL conical tube | VWR | 490001-621 | |
| cell scraper | Denville Scientific | TC9310 | |
| 1.5 mL Microcentrifuge tubes | USA Scientific | 1615-5500 | |
| HCl | Fluka Analytical | 318949-1L | |
| NaOH | J.T. Baker | 5674-02 | |
| Wooden applicators | Solon Care | 55900 | |
| Eppendorf microcentrifuge 5424 | Fisher Scientific | 05-400-005 | microcentrifuge |
| Sorvall ST16R | Thermo Fisher Scientific | 75004381 | benchtop centrifuge |
| Amersham ECL Rabbit IgG, HRP-linked whole Ab (from donkey) | GE Healthcare | NA934-1ML | Secondary Antibody |
| Amersham ECL Mouse IgG, HRP-linked whole Ab (from sheep) | GE Healthcare | NA931-1ML | Secondary Antibody |
| SuperSignal West Pico Chemiluminescent Substrate | Thermo Fisher Scientific | 34080 | ECL detection solution |
| Isotemp Incubator | Thermo Fisher Scientific | Incubator | |
| Mutitron 2 | INFORS HT | Shaking incubator | |
| Isotemp Digital-Control Water Bath Model 205 | Fisher Scientific | water bath | |
| Y2H mouse cDNA library in Y187 (pan tissue) | Clontech | 630482 | commercially available cDNA Library |
| Y2H mouse cDNA library in Y187 (mouse brain) | Clontech | 630488 | commercially available cDNA Library |
| pGADT7 AD Vector | Clontech | 630442 | commercially available AD Vector housing many cDNA libraries |
| pGBKT7 DNA-BD Vector | Clontech | 630443 | commercially available DNA-BD Vector |
| Biolase DNA Polymerase | Bioline | BIO-21042 | DNA polymerase used for section 2.4 |
| GeneMate GCL-60 Thermal Cycler | BioExpress | P-6050-60 | pcr machine |
| TempAssure 0.5 mL PCR tubes | USA Scientific | 1405-8100 |
References
- Fields, S., Song, O. A novel genetic system to detect protein-protein interactions. Nature. 340 (6230), 245-246 (1989).
- Bruckner, A., Polge, C., Lentze, N., Auerbach, D., Schlattner, U. Yeast two-hybrid, a powerful tool for systems biology. International Journal of Molecular Sciences. 10 (6), 2763-2788 (2009).
- Vidal, M., Fields, S. The yeast two-hybrid assay: still finding connections after 25 years. Nature Methods. 11 (12), 1203-1206 (2014).
- Pashkova, N., et al. DEEPN as an Approach for Batch Processing of Yeast 2-Hybrid Interactions. Cell Reports. 17 (1), 303-315 (2016).
- Rajagopala, S. V. Mapping the Protein-Protein Interactome Networks Using Yeast Two-Hybrid Screens. Advances in Experimental Medicine and Biology. 883, 187-214 (2015).
- Weimann, M., et al. A Y2H-seq approach defines the human protein methyltransferase interactome. Nature Methods. 10 (4), 339-342 (2013).
- Yachie, N., et al. Pooled-matrix protein interaction screens using Barcode Fusion Genetics. Molecular Systems Biology. 12 (4), 863 (2016).
- Trigg, S. A., et al. CrY2H-seq: a massively multiplexed assay for deep-coverage interactome mapping. Nature Methods. , (2017).
- Estojak, J., Brent, R., Golemis, E. A. Correlation of two-hybrid affinity data with in vitro measurements. Molecular and Cellular Biology. 15 (10), 5820-5829 (1995).
- Rajagopala, S. V., Hughes, K. T., Uetz, P. Benchmarking yeast two-hybrid systems using the interactions of bacterial motility proteins. Proteomics. 9 (23), 5296-5302 (2009).
- James, P., Halladay, J., Craig, E. A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 144 (4), 1425-1436 (1996).
- Barbas, C. F., Burton, D. R., Scott, J. K., Silverman, G. J. Quantitation of DNA and RNA. CSH Protoc. 2007, pdb ip47 (2007).
- Sambrook, J., Russell, D. W. SDS-Polyacrylamide Gel Electrophoresis of Proteins. Cold Spring Harbor Protocols. 2006 (4), (2006).
- Towbin, H., Staehelin, T., Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences USA. 76 (9), 4350-4354 (1979).
- Alegria-Schaffer, A. Western blotting using chemiluminescent substrates. Methods in Enzymology. 541, 251-259 (2014).
- Lee, P. Y., Costumbrado, J., Hsu, C. Y., Kim, Y. H. Agarose gel electrophoresis for the separation of DNA fragments. Journal of Visualized Experiments. (62), (2012).
- Harper, J. W., Adami, G. R., Wei, N., Keyomarsi, K., Elledge, S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 75 (4), 805-816 (1993).

