Rat Model of Widespread Cerebral Cortical Demyelination Induced by an Intracerebral Injection of Pro-Inflammatory Cytokines
Summary
The protocol presented here allows the reproduction of a widespread grey matter demyelination of both cortical hemispheres in adult male Dark Agouti rats. The method comprises of intracerebral implantation of a catheter, subclinical immunization against myelin oligodendrocyte glycoprotein, and intracerebral injection of a pro-inflammatory cytokine mixture through the implanted catheter.
Abstract
Multiple sclerosis (MS) is the most common immune-mediated disease of the central nervous system (CNS) and progressively leads to physical disability and death, caused by white matter lesions in the spinal cord and cerebellum, as well as by demyelination in grey matter. Whilst conventional models of experimental allergic encephalomyelitis are suitable for the investigation of the cell-mediated inflammation in the spinal and cerebellar white matter, they fail to address grey matter pathologies. Here, we present the experimental protocol for a novel rat model of cortical demyelination allowing the investigation of the pathological and molecular mechanisms leading to cortical lesions. The demyelination is induced by an immunization with low-dose myelin oligodendrocyte glycoprotein (MOG) in an incomplete Freund's adjuvant followed by a catheter-mediated intracerebral delivery of pro-inflammatory cytokines. The catheter, moreover, enables multiple rounds of demyelination without causing injection-induced trauma, as well as the intracerebral delivery of potential therapeutic drugs undergoing a preclinical investigation. The method is also ethically favorable as animal pain and distress or disability are controlled and relatively minimal. The expected timeframe for the implementation of the entire protocol is around 8 – 10 weeks.
Introduction
MS is an immune-mediated, inflammatory disease of the CNS, which primarily damages myelin sheets, but eventually leads to the axonal loss and permanent neuronal damage. MS is the most common immune-mediated disease of the CNS with an estimated prevalence of about 2.3 million people worldwide, according to the National MS Society1, and represents a major personal and socioeconomic burden. The average age of the disease onset is 30 years and leads to the loss of productive years by causing severe disability. MS is currently incurable, and the present treatment modalities aim to manage the symptoms during an acute relapse in relapsing-remitting MS and to modify the course of the disease to decrease the relapse frequency by immunomodulatory therapy2,3. No treatment option has yet proven efficacious for the progressive types4, with the exception of a recent clinical trial of B-cell depleting therapy, which was shown to be efficacious in a subgroup of primary progressive MS (PPMS) patients with active inflammation5. Whilst several potential genetic6 and environmental risk factors7 have been identified, the etiology of MS, however, remains unknown.
MS is characterized by large inflammatory demyelinating plaques in, and diffuse injuries to, the white matter8,9. Focal lesions have been associated with an extensive T-cell mediated attack, oligodendrocyte destruction, reactive astrogliosis, and axonal degeneration, leading to a motor neuron decline. Grey matter demyelination and atrophy have gained recognition as additional histopathological features of the disease9,10,11. The latter has been suggested to contribute to the neurological dysfunction and cognitive decline in patients12,13. Three patterns of cortical demyelination have been distinguished, namely i) leukocortical, contiguous with the white matter lesions (34%), ii) small, perivascular (16%), and iii) subpial (50%). Unlike focal white matter lesions, these grey matter lesions have been reported to lack a T-cell mediated attack and are instead characterized by an enhanced microglial activation, apoptosis, and neuronal loss12.
To date, it has not been possible to recapitulate human MS in a single animal model, largely due to the complexity of the disease. A variety of MS animal models, each simulating different aspects of disease pathogenesis and progression have, instead, been developed14,15. Current animal models mimic three different disease processes: i) focal inflammatory lesions, ii) diffuse white matter injury, and iii) diffuse grey matter pathology.
Animal studies of MS white matter plaques have mostly been conducted in rodent encephalomyelitis (EAE) models. Test animals are actively immunized with an emulsion containing a myelin antigen [usually myelin oligodendrocyte glycoprotein (MOG)16,17, myelin basic protein (MBP)18, or proteolipid protein (PLP)19], together with a complete Freund's adjuvant (CFA)20. The disease can also be passively induced by an adoptive transfer of myelin-specific T-cells21. The disease course depends on the antigen/mouse strain combination that is used. MOG35-55 in C57BL/6, for instance, results in a monophasic chronic disease22, whilst PLP139-151 in Swiss Jim Lambert (SJL) mice leads to a relapsing-remitting disease course23 in a gender-specific manner24. Rat MOG35-55, further,induces an encephalitogenic T-cell response, whilst human MOG35-55 induces B-cell-dependent inflammation in C57BL/6 mice25. Various EAE models provide an excellent tool to study cell-mediated inflammation primarily in the spinal cord and cerebellum, but forebrain structures like the cortex, corpus callosum, and subcortical structures remain largely spared26. Neither diffuse white matter injury nor grey matter demyelination is, furthermore, adequately replicated in EAE models26,27. Cortical demyelination associated with active EAE induction has been reported in marmosets28,29 and in certain Lewis rat sub strains, in the latter case attributed to the prevailing combinations of MHC class I and class II isotypes and alleles30.
The cuprizone model31 is a useful tool for studying diffuse white matter demyelination and grey matter pathology with an extensive demyelination of the cortical, sub-cortical32, and hippocampal33 regions, as well as the corpus callosum34 and the caudate putamen35. Cuprizone intoxication, which, in principle, results in metabolic stress-induced apoptosis of the oligodendrocytes, mimics some characteristics of the cortical demyelinating lesions of MS brains such as a microglia activation, astrogliosis, and a relative lack of infiltrating peripheral immune cells. The lack of neuronal apoptosis and thalamic atrophy, as well as the complete resolution of demyelination with a robust remyelination observed upon the cessation of dietary cuprizone supplementation32, however, limit the cuprizone intoxication's usage as a preclinical MS model. Toxic demyelination can also be induced by a focal injection of lysolecithin or ethidium bromide into the white matter tracts36,37, but these methods are rarely used. Toxic demyelination models are especially suitable for the analysis of the complex mechanisms of remyelination, such as the requirement for oligodendrocyte progenitor cells and astrocytes38,39. Detailed information on EAE and intoxication models are provided in two recent reviews15,40.
The cytokine-induced demyelination was initially developed to study spinal white matter lesions in EAE41. Later, it was modified to study cortical grey matter pathologies in MS. Dark Agouti (DA) or Lewis rats are first primed by a sub-clinical immunization with MOG1-12542,43 or MOG1-11644 in an incomplete Freund's adjuvant (IFA). Unlike classical EAE models, these primed animals do not exhibit clinical symptoms of focal inflammatory lesions in the spinal cord. Instead, an inflammatory response and demyelination in the brain are subsequently achieved by an intracerebral administration of a pro-inflammatory cytokine mixture [tumor necrosis factor alpha (TNFα) and interferon gamma (IFNγ)] once the animals have attained a stable titer of anti-MOG antibodies in the blood.
Studies by Merkler et al.42 and Gardner et al.44 have proven the efficacy of a subpial cortical demyelination induction by a sub-clinical MOG immunization and an intracerebral or subarachnoidal cytokine injection. The reported duration of the demyelination was, however, too short-a complete remyelination occurred in 14 days or less, thereby limiting the window for any pharmacological intervention testing. Both models, moreover, utilize a traumatic injection modus, which could per se cause an injection trauma and a blood-brain barrier (BBB) breakdown and thus lead to an uncontrolled recruitment of inflammatory cells into the parenchyma. Both studies, furthermore, demonstrated demyelination that is restricted to a limited area, in the ipsilateral cortex, or in the close vicinity of the site of the cytokine injection.
To overcome these limitations, we implanted a catheter in the right parietal cortex of DA rats, with the catheter tip located just above the corpus callosum. To allow a full recovery of the BBB integrity, the animals were allowed a resting period of 2 weeks after the catheter implantation. Subsequently, the rats were sub-clinically immunized with 5 µg of recombinant MOG1-125 in IFA. Following the attainment of a stable anti-MOG antibody titer after around 4 weeks, 2 µL of cytokine mixture was injected via the catheter within 10 min using a programmable syringe pump. This procedure elicited a widespread cortical demyelination of both the ipsi- and the contralateral cerebral hemispheres in 15 days, with a partial remyelination around 30 days post-cytokine injection43. Multiple demyelination phases could, moreover, be induced by the repeated administration of pro-inflammatory cytokines through the catheter, and global brain atrophy, a common feature of progressive MS subtypes45, could be induced as early as after the second demyelination phase43. Importantly, the implanted catheter could also be used to test pharmacological interventions.
The protocol described below provides a detailed explanation of the experimental steps for a reproducible generation of widespread cortical demyelination in both cerebral hemispheres of DA rats using an intracerebral catheter.
Protocol
All methods described here have been approved by local authorities (Bundesministerium für Wissenschaft und Forschung (Austrian Ministry of Science and Research); License Number: 66.010/0132-WF/V/3b/2014). The adult male DA rats (10 – 12 weeks of age) were housed in a 12/12 h light/dark cycle with free access to food and water.
1. Material Preparation
NOTE: The surgery is conducted under aseptic conditions. Prior to start, make sure that all surgical instruments including the drill bits are cleaned with an appropriate disinfectant.
- Prepare an anesthetic mixture: 0.02 mg/mL of fentanyl, 0.4 mg/mL of midazolam, and 0.2 mg/mL of medetomidine (final concentrations in the mixture).
NOTE: See the respective Discussion section for alternative anesthetics. - Prepare an antidote mixture: 0.07 mg/mL of flumazenil and 0.42 mg/mL of atipamezole (final concentrations in the mixture).
- Assemble the catheter and the catheter cap with the inlet and the screw. Cut the catheter to a length of 2 mm with a scalpel (Figure 1).
NOTE: Do not use scissors for this, since they squeeze and distort the circular cross-sectional shape of the catheter tip.
2. Surgical Preparation
- Anesthetize the rat by an intraperitoneal (i.p.) administration of the anesthetic mixture (1.5 mL/kg of body weight).
- Shave the head of the rat between the ears using the electric shaver. Place a homeothermic blanket on the stereotactic frame before positioning the animal, to avoid hypothermia throughout the surgery.
- Immobilize the rat's head in the stereotactic frame using the ear bars and bite plate, ensuring that the head is horizontal and stable. Check the stability by applying pressure to the skull with finger or forceps.
NOTE: A loose fixation and non-horizontal positioning within the stereotactic frame may cause a deviation from the intended coordinates. - Apply lubricating eye drops to prevent cornea dryness during the surgery. Cover the eyes with an opaque material to prevent any surgical light exposure.
- Clean the shaven area by alternating the application of 70% ethanol and 10% povidone-iodine complex.
NOTE: Follow all precautionary measures during surgery to the avoid infection. The surgery is conducted under aseptic conditions. If asepsis is broken, then the contaminated material has to be replaced.
3. Catheter Implantation
- Make a longitudinal incision of about 2 cm of length in the middle of the head skin. Use bulldog clamps to hold the skin off to the sides. For an overview of these steps, see Figure 2.
- Remove the blood using a cotton-tipped applicator.
- Remove the skull periosteum. Clean the tissue with the cotton-tip applicator and expose the skull bone. Allow the skull to dry for about 1 min.
- Identify the anatomical landmarks, Lambda, Bregma, and medial suture. With the drill installed on the stereotactic frame, position the drill tip at the Bregma as the starting point. Move 2 mm posterior from the Bregma and move ~2.4 mm laterally to the medial suture.
- Drill a 0.5 mm diameter hole for the catheter at this position. Gently puff away any bone dust.
NOTE: It is important that the dura mater remains intact during the drilling. To ensure this, 1) use a drill that can be installed on the stereotactic frame, 2) inspect the hole frequently during the drilling, and 3) drill down in small steps-if too much pressure is applied to the skull, the drill tip will continue into, and damage the brain when the skull is fully penetrated. - Drill 3 further holes (~1.3 mm in diameter) for the anchor screws a few millimeters away from the first hole. Gently blow bone dust away.
NOTE: Select anchor screw locations that provide enough space for the catheter top (~2 mm in diameter) and the anchor screw tops (~1 mm). - Remove the bone dust by irrigation with around 1 – 2 mL of sterile phosphate-buffered saline (PBS) or physiological saline using a syringe. Clean the skull. Tighten the anchor screws by 2 – 3 full turns.
NOTE: The anchor screws are necessary to stabilize the set-up by holding the dental cement, and, thereby, the catheter, in place. Whilst tightening an anchor screw, ensure that it is not easily removable by gently lifting it upwards with forceps. Since the implantation of the catheter itself causes tissue trauma, additional dura injury whilst drilling for, or tightening the anchor screws will lead to multiple traumatic injuries, and possibly hamper the comparability within a group. Tighten the anchor screws first and insert the catheter last. - Insert the 2-mm length catheter through the first hole, perpendicular to the skull surface. While still holding the catheter in, apply a little dental cement and let it polymerize with a brief (~5 s) exposure to the dental curing light to stabilize the catheter, allowing the use of both hands in the next step.
CAUTION: While working with a dental curing light, avoid looking directly at the tip, or at the light reflected from the application area, as the high intensity of this light can cause retinal damage. Use appropriate protective goggles. - Apply more dental cement around the catheter, anchor the screws, and solidify the dental cement with the dental curing light (~15 – 30 s). Confirm the hardening of the cement with the tip of a forceps.
4. Closing of the Wound and Antagonization of Anesthesia
- Close the head skin with resorbable sutures, anterior and posterior to the catheter.
NOTE: Since there will be a bumpy set-up over the skull at the end of the implantation, do the wound closure accordingly. Lifting up the skin too much will result in discomfort for the animal. - Inject the antidote mixture subcutaneously (1.5 mL/kg of body weight) using a 1 mL syringe with a 26 G needle.
- Administer enrofloxacin (2.5%) by subcutaneous injection (7.5 mg/kg body weight) for prophylactic antibiotic treatment. Administer carprofen (1 mg/mL; 5 mg/kg body weight) and buprenorphine (1.2 mg/kg) for pain relief by subcutaneous injection.
5. Post-operative Care and Medication
- Return the animal to the modified cage and keep it under observation for 1 – 3 h, with an application of infrared light to avoid hypothermia. Constantly observe and reposition the animal every 5 to 10 minutes until post-operative recovery. Take special care to avoid constant light exposure to the eyes till recovery.
- Repeat enrofloxacin (7.5 mg/kg body weight) and carprofen (1 mg/mL; 5mg/kg body weight) administrations by subcutaneous injections the day after surgery. Buprenorphine is not necessary to be refreshed as the previous treatment is effective for 72 hours.
6. Preparation of Immunization Mixture (at the Earliest 14 Days after Catheter Implantation)
Note: Place the syringes on ice during the preparation procedure.
- Connect two 10 mL Luer lock tip glass syringes to the short arms of a 3-way stopcock and close the third outlet with the long arm.
- Ensure the connections are secure and leak-free: add approximately 4 mL of sterile PBS to the open syringe while holding piston 2. Insert piston 1 and push both pistons back and forth whilst checking for leakage. If no leakage occurs, discard the PBS and remove piston 1 again.
- Pipette 1 mL of IFA and 50 µg of rMOG1-125 together and adjust the mixture to a final volume of 2 mL with sterile PBS (pH 7.4) in a suitable tube.
NOTE: Due to losses of emulsion on the tips or walls of syringes during the preparation, prepare a larger volume than intended for the administration. It is similarly more practical to prepare for more than 1 animal at once. - Place the diluted IFA and rMOG1-125 mixture in the open syringe. Insert the piston gently whilst maintaining a loose pressure on the opposite piston (Figure 3A).
- Emulsify the inoculum by driving it from one syringe to the other by pushing the pistons back and forth, until it is white and viscous (Figure 3B).
- Fix a 1 mL Luer lock syringe to the open short arm of the 3-way stopcock and fill it with inoculum (Figure 3C). Distribute all inoculum to 1 mL syringes. Keep it on ice until the injection. Administer the mixture on the day of the preparation.
7. Immunization
- Anaesthetize the rat with isofluorane in a chamber (~2 min, mixed with oxygen 2 L/min) and then sustain anesthesia through a mask (mixed with oxygen 1.5 L/min).
- Inject 200 µL of inoculum subcutaneously at the tail base using a 21 G needle.
NOTE: Administer the injection slowly as the solution is viscous.
8. Determination of Antibody Titers
- Withdraw ~200 µL of blood 4 weeks after the immunization in order to determine the anti-MOG antibody titers.
- Coat the wells of a 96-well plate with MOG (5 µg/mL in PBS) and incubate them for 1 h at 37 °C.
- Block the plate with 1% bovine serum albumin (BSA) in PBS for 1 h at room temperature.
- Incubate the plate with rat serum (1:50) and standard it for 2 h at 37 °C. Wash the plate 3x with 200 µL of PBS/Polysorbate 20.
- Incubate the plate with horseradish peroxidase-conjugated anti-rat IgG secondary antibody (1:10,000). Wash the plate 3x with 200 µL of PBS/Polysorbate 20.
- Add 100 µL of peroxidase substrate solution per well and incubate it for 20 – 30 min in the dark at room temperature.
- Measure the optical density at a 405 nm wavelength and calculate the antibody titer from the optical density using a standard curve.
9. Intracerebral Cytokine Injection
- Adjust the length of the connector cannula (2 mm). (See Figure 4 for the preparation steps.)
- Fill a 1 mL syringe with the cytokine mixture (500 ng/µL of TNF-alpha, 300 U/µL of recombinant rat IFN-gamma in sterile PBS). Connect the syringe to a connector cannula. Fill the cannula with the cytokine mixture. Avoid any bubbles.
- Mount the syringe onto the programmable syringe pump and program it to inject 0.2 µL/min (Figure 5A). Start the pump and keep it working in order to avoid an air bubble formation at the tip of the cannula.
NOTE: The injection speed must take into account the inner diameter of the specific syringe used; thereby, the syringe diameter has to be registered during the pump set up. - Anesthetize the rat with isoflurane in a chamber (~2 min, mixed with 2 L/min of oxygen) and then sustain the anesthesia through a mask (mixed with 1.5 L/min of oxygen) (Figures 5B and 5C). Apply lubricating eye drops as the animal will be anesthetized for at least 30 min.
- Remove the catheter cap with the inlet. Insert the connector cannula into the catheter and screw and tighten it (Figures 5D and 5E).
NOTE: Do not overtighten it, as this will destroy the upper tip of the catheter. - Allow the injection to proceed for 10 min (the total volume of injection being 2 µL). Stop the pump. Leave the cannula inside the catheter for 20 min to allow the injected volume to fully diffuse.
- Unscrew the connector cannula and remove it slowly to avoid a vacuum effect.
- Reattach the catheter cap with the inlet and screw it. Allow the animal to recover from the anesthesia in a cage.
Representative Results
Cortical demyelination could be assessed at different time points after a cytokine injection by immunohistochemistry for proteolipid protein (PLP) (Figure 6). Figure 6A shows intact PLP immunoreactivity at day 15 in a MOG-immunized control animal that received only sterile PBS through the implanted catheter. On day 1 after the cytokine injection, demyelination could already be detected in MOG-primed animals, albeit only in the close vicinity of the catheterized area (Figure 6B). The PLP immunoreactivity stays intact in the contralateral cortex 1-day post-cytokine injection. On day 3, a gradual increase in the loss of the PLP immunoreactivity, which spreads in the ipsilateral cortex (Figure 6C), could be observed. Contralateral cortical demyelination could also be detected at day 3 (Figure 6D), but it is rather restricted to the area beneath the anchor screws, possibly due to a low-flow area of interstitial fluid caused by the anchor screws43. The absence of a similar observation in the PBS-injected control animals excludes the possibility of trauma-induced demyelination stemming from the anchor screw.
Between days 9 – 15, demyelination affects large parts of the cortex of both hemispheres (Figures 6E, 6F, and 6G). This coincides with an observation of slow behavior, though without a statistically significant decrease in motor skills in a rotarod test43. The cortical demyelination is sustained for up to 30 days post-cytokine injection in both hemispheres (Figures 6H and 6I) with only a partial remyelination. Figure 6J shows a quantification of PLP loss in the cortical grey matter after the intracerebral cytokine injection. It should be noted that PLP immunoreactivity has not yet been assessed after periods longer than 30 days; thereby, the instantaneous resolution of remyelination, if there is any, remains to be assessed by further experimentation. A second administration of cytokine mixture through the implanted catheter 30 days after the first injection results in marked brain atrophy at day 15 (Figure 7).

Figure 1: Preparation of the catheter. (A and B) The guide cannula and the dummy cannula (catheter cap with inlet) are assembled and screwed. (C) Then the catheter is cut to 2 mm in size with the help of a scalpel. The microscopic observation showed that the usage of scissors for that purpose distorts the circular shape of the cannula tip, and, thereby, must be avoided. Please click here to view a larger version of this figure.
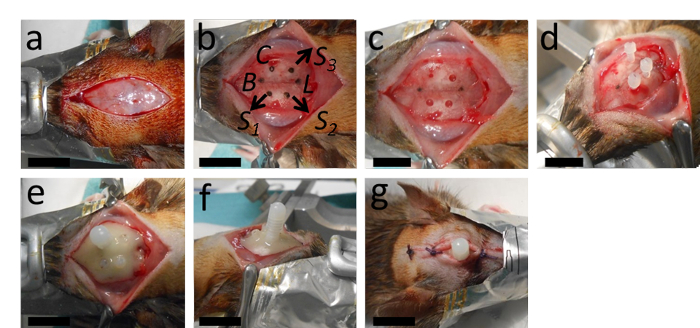
Figure 2: The implantation of the catheter. (A) The surgery starts with a longitudinal incision and removal of the periosteum. (B, C) This panel shows the marking of the place for the catheter at 2 mm posterior from Bregma and 2.4 mm lateral to the right from the sagittal suture; as well as the places for the holes intended for the three anchor screws with an appropriate distance from the catheter and Lambda. (D) After drilling the catheter hole (a 0.5 mm diameter, with a round drill tip) and the holes for the anchor screws (1.3 mm-diameter with a twisted drill tip), the anchor screws are tightened. (E, F) Then the catheter is inserted and the whole setup is stabilized with polymerizing dental cement. (G) The wound is stitched with two or three knots anterior and posterior to the catheter. B = Bregma; L = Lambda; C = Catheter; S1, S2 and S3 = places for the holes for the three anchor screws. The scale bars = 1 cm. Please click here to view a larger version of this figure.
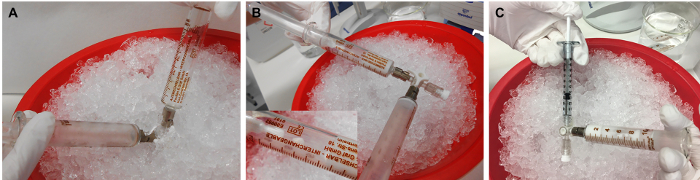
Figure 3: Preparation of rMOG/IFA emulsion. (A) The mixture of rMOG, PBS, and IFA is emulsified by pressing the inoculum from one syringe to another by pushing the pistons back and forth, (B) until it is white and viscous. (C) Subsequently, the inoculum is distributed to 1 mL syringes for the injection. 5 µg of rMOG is used in 200 µL of PBS/IFA mixture to sub clinically immunize one rat; however, due to the losses at the tips and walls of the syringes during the preparation, a larger volume should be prepared. Please click here to view a larger version of this figure.
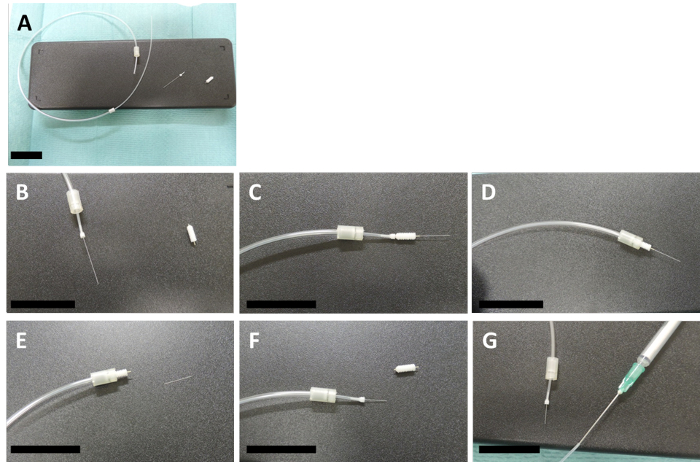
Figure 4: Preparation of the connector cannula. (A – D) These panels show how a connector and an internal are assembled with a 2-mm size template guide cannula. (E) The internal is cut to the same size as the guide cannula with the help of a scalpel (F) and the template guide is then unscrewed. (G) The other end of the connector cannula is fixed to a 1 mL syringe, which contains the injection mix, with a 20 G needle. The scale bars = 3 cm. Please click here to view a larger version of this figure.
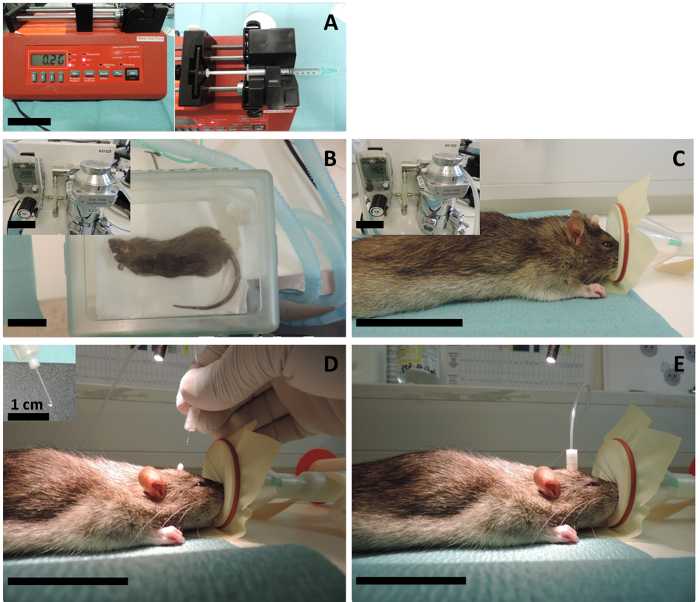
Figure 5: Intracerebral injection. (A) A programmable syringe pump is adjusted for a 2 µL/min injection speed, and the 1 mL syringe filled with cytokine mix (or sterile PBS for the controls) is mounted to the pump. (B) The animal is first anesthetized in the chamber using 5% isoflurane with a 2 L/min oxygen flow and then (C) the anesthesia is sustained through the mask using 2.5% isoflurane with a 1.5 L/min oxygen flow. (D) The catheter cap with the inlet (the dummy cannula) is screwed off and the injection cannula is inserted through the implanted catheter. Since the volume of the injection is very small, the investigator should be cautious to avoid air bubbles at the tip of the cannula. For that reason, it is important to start the insertion while the pump is in operation and only when there is a growing liquid bubble at the tip. The extra volume will not go into the brain anyway, as it breaks down on top of the catheter before the insertion. (E) Then the connector cannula is tightened, and the pump is let to operate for 10 min. After 10 min of injection, the pump is stopped, and the cannula is left inside for 15 – 20 min to allow for the diffusion of the injected volume to the interstitial fluid. The scale bars = 5 cm, unless indicated otherwise. Please click here to view a larger version of this figure.
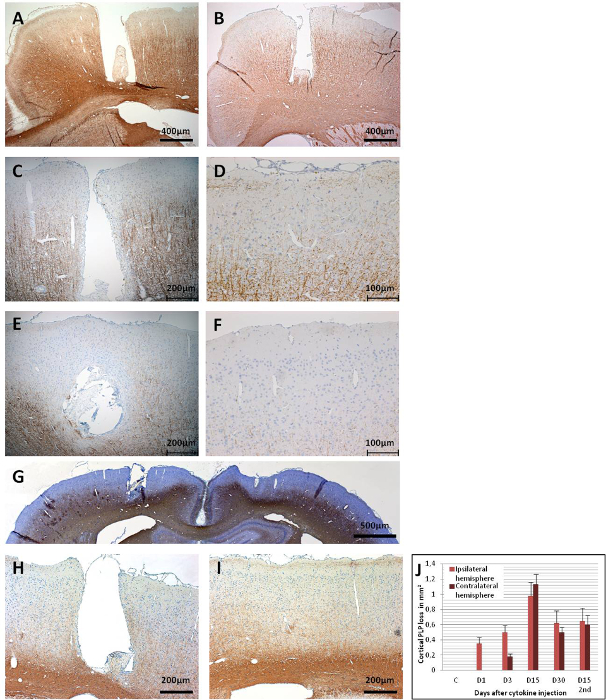
Figure 6: PLP immunoreactivity in coronal brain sections. (A) This panel shows a control brain (MOG-primed) with a PBS injection (day 15), which does not result in a cortical demyelination. (B) As early as day 1 post-cytokine injection, demyelination is apparent in the catheter area. (C) A broader loss of PLP immunoreactivity is observed in the ipsilateral cortex at day 3, (D) as well as at the contralateral side. Widespread loss of PLP immunoreactivity is observed in both hemispheres at day 15, as (E) this panel shows the ipsilateral cortex and (F) this panel shows the contralateral cortex. (G) An overview of both hemispheres is given in showing the widespread loss of PLP at day 15. At day 30, as (H) this panel shows the ipsilateral cortex and (I) this panel shows for contralateral cortex, there is still remarkable demyelination, but also some remyelinated areas could be observed. (J) This panel shows the quantification of the demyelination (PLP loss in mm2/hemisphere). 1.5 – 2 µm coronal brain sections were used for the PLP detection with MS anti-PLP with a dilution factor of 1:500. The sections were counterstained with hematoxylin for cell nuclei. For detailed information on the immunohistochemistry, see Ucal et al.43. Panel G and J were modified from Ucal et al.43. Please click here to view a larger version of this figure.
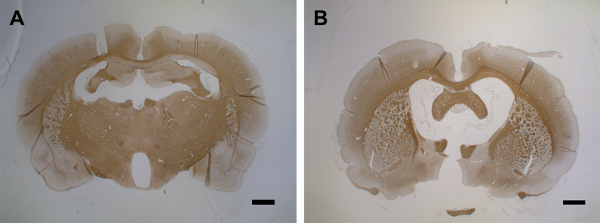
Figure 7: Brain atrophy after second cytokine injection. (A) This panel shows a control brain (MOG-primed) with a PBS injection (day 15). (B) At day 15 after the first cytokine injection, a second injection leads to brain atrophy within 15 days. 1.5 – 2 µm coronal brain sections were used for the PLP detection with MS anti-PLP with a dilution factor of 1:500. The sections were counterstained with hematoxylin for cell nuclei. For detailed information on immunohistochemistry, see Blakemore37. The scale bars = 500 µm. Please click here to view a larger version of this figure.

Figure 8: Modified cages to prevent catheter removal by the animal. In the standard cages, the food holder space on the grid is located closer to the cage bottom, creating a risky narrow space that increases the chance of the catheter to tangle with the grid and, thereby, its removal. To avoid this, the cage has to be modified. This narrow space was blocked with a transparent plane, allowing the observance of the animal. The food has to be given inside the cage in these modified cages. Please click here to view a larger version of this figure.
Discussion
Our method uses DA rats. The adaptation to mice will likely require the use of a smaller catheter and screws. It should also be borne in mind that the disease course, the inflammatory response, and the extent of demyelination might differ from what is presented here if a different species/strain is used. Such differences have been observed with classical EAE models using different strains of mice. MOG92-106 of rat origin, for instance, resulted in primary progressive or secondary progressive EAE in A.SW mice, whilst it induced relapsing-remitting EAE in SJL/J mice46. Animals of the same strain should, therefore, be used. Gender differences in EAE manifestation have also been reported in various previous studies24. The occurrence of such gender effects might well be expected for the protocol described here yet remains to be validated in further experiments.
The intraperitoneal (IP) administration of an anesthetics mixture comprising 0.02 mg/mL of fentanyl, 0.4 mg/mL of midazolam, and 0.2 mg/mL of medetomidine is used for the surgical intervention. Adult male DA rats weighing 270 – 300 g require around 0.4 – 0.6 mL of this mixture (i.e., ~1.5 mL/kg) to induce an anesthesia lasting 60 – 90 min. Following the surgery, the anesthesia is antagonized by a subcutaneous injection of an antidote comprising 0.07 mg/mL of flumazenil and 0.42 mg/mL of atipamezole in physiological saline (0.9% NaCl). A dose of 1 – 1.5 mL/kg antagonizes the anesthesia within 5 min. Alternatively, the animals can be allowed to wake up spontaneously upon a physiological washing out of the anesthetics, but in that case, the animals would need to be kept under observation until they are fully conscious.
Other anesthesia options frequently used for animal surgeries, such as an IP injection of ketamine and xylazine47 or sodium pentobarbital48, or an inhalation of volatile anesthetics like isofluorane49 and halothane50, can also be considered for the surgery presented here. It is critical, however, to choose an anesthetic agent that does not interfere with the intended downstream intervention(s).
During the immunization and intracerebral cytokine injection, 5% isoflurane is used for the anesthesia. The model described here was established using rats, and the experimental details listed are, thus, specifically applicable to the rat. The catheter implantation coordinates were selected to enable the simultaneous analysis of possible white matter changes (the catheter tip in the corpus callosum). Whilst the catheter insertion site can be varied with respect to the anteroposterior and lateral position, the selection of the central sulcus requires the avoidance of damage to the superior sagittal sinus.
A further feature of the described method is the equivalent demyelination of both ipsi- and contralateral hemispheres, possibly resulting from the carriage of the injected cytokine mixture to the subarachnoid space by the physiological flow of the interstitial fluid from cortical regions51. The injection mode, and not the location of the catheter, therefore, causes demyelination throughout the cerebral cortex, and the choice of right or left parietal cortex should, thus, be immaterial in this regard.
The protocol uses a 26 G catheter, which is small enough to avoid extensive traumatic injury and large enough to avoid an increased rate of clogging of the catheter tip over the long course of the experiment. Certainly, the implantation and the presence of the catheter itself cause an astrocytic and microglial activation, also in the control animals receiving only the catheter implantation; however, this is minor when compared to the cytokine-injected animals.43 To avoid any interference with subsequent analyses, we used MRI-compatible catheters made of poly-ether-ether-ketone (PEEK).
A similar depth of demyelination is, in fact, created in both ipsi- and contralateral regions with the presented method. This implies that the catheter depth/length might not play a major role in the pattern and extent of demyelination in the cortex. Therefore, a modification of the catheter length might be considered in order to reduce the catheter-induced lesion size. Nevertheless, a significantly shorter catheter length might cause a slightly less pronounced cortical demyelination, whilst a conclusive answer would only be obtained by experiments specifically testing for the catheter length.
One advantage of the model is that the implanted catheter allows for the testing of potential therapeutics administered to the cortex via the catheter to allow remyelination at or after the peak of histologically detectable cortical demyelination (day 15 or later), whilst in a pretreatment setting this would be after the immunization but before the cytokine injection. The decision on the time frame when therapeutics would be administered, therefore, will depend on the particular research question and the drug of interest.
Following catheter implantation, it is important to house animals single in the modified (preferably high top) cages in order to avoid catheter removal till the end of the study (Figure 8). Animals might also unscrew the catheter cap with the inlet, although this rarely happens. The animals should be observed daily and removed caps should be replaced with fresh ones, to avoid catheter tip blockage in the absence of an inlet, and to ensure an accurate delivery into the parenchyma following the intracerebral injection. The animals are immunized at the earliest 2 weeks after the catheter implantation to allow the healing and closure of the blood-brain barrier.
Serum anti-MOG antibody titers should be measured after the immunization. A dose-response experiment showed that 5 µg of MOG1-125 (in IFA) provided sufficient immunization within 4 weeks in adult male DA rats. A titer of 5,000 µg/mL and higher would be sufficient, but will certainly depend on several factors, including the MOG preparation and the animal strain and, thus, will have to be determined individually. It is important to avoid excessively high antigen doses potentially resulting in a classical EAE phenotype with paralyzed hind limbs even before the cytokine injection.
Each animal is immunized with 5 µg of recombinant myelin oligodendrocyte glycoprotein (rMOG1-125) emulsified in 200 µL of incomplete Freund's Adjuvant (IFA). Since some of the emulsion is lost within the syringe during the preparation, it is advisable to prepare more than this amount for each animal. We used recombinant MOG (1-125 from the N-terminus of rat MOG), which was expressed in Escherichia coli and was then purified to homogeneity by chelate chromatography, dissolved in 6 M urea, and dialyzed against PBS to obtain a physiological preparation52,53. Commercially available MOG may, however, also be used.
Other antigen preparations, such as MOG1-116, MOG35-55 or PLP139-151 are used in various EAE models, and antigen and animal strain differences are known to induce distinct disease phenotypes in these models20. These antigen preparations were not tested in DA rats and, if used in preference to rMOG1-125, might induce a disease phenotype or histology results differing from what is presented here.
A connector cannula the same length as the catheter is prepared prior to the intracerebral injection. This can be done by assembling it with a template catheter and cutting it to the same size (2 mm in length) (Figure 4). It is important that the connector cannula be air bubble-free during the cytokine injection-because the injection volume is only 2 µL, even a tiny air bubble at the cannula tip will significantly reduce the volume of the liquid successfully delivered into the brain. This is achieved by keeping the pump running and inserting the cannula only when a growing drop of injection liquid is present at the tip. Following the cannula insertion, the connector is screwed to the catheter while avoiding overtightening so as not to damage the upper tip of the catheter, which will make it difficult to recap after the injection. An injection speed of 0.2 µL/min is used to avoid injection-induced trauma. Moreover, a slow injection, combined with a 20 min waiting period after the injection, ensures the diffusion of the injected liquid into the interstitial fluid and an effective draining into the CSF. The cannula is then removed slowly to avoid a vacuum effect.
The reported method includes surgical intervention and, therefore, requires staff able to perform stereotactic survival surgery. Personnel in direct contact with the animals should have taken the appropriate animal experimentation courses. The remainder of the protocol can be carried out by competent lab members.
The method is intended to produce inflammation-triggered demyelination of the cerebral cortex and does not reproduce all features of human MS (e.g., the occurrence of focal inflammatory white matter lesions, which is a hallmark of human MS).
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank all personnel of the Institute of Biomedical Research at the Medical University Graz for their help and cooperation, as well as Christopher John Wrighton for proofreading the manuscript.
Materials
| Adult male Dark Agouti rats (300 ±25 g) | |||
| Fentanyl | Hameln pharma plus, Germany | as Fentanyl-Citrate, 50 µg/ml | |
| Midazolam | ERWO Pharma, Austria | 50039017 | 5 mg/ml |
| Medetomidin | Orion Pharma, Finland | as Medetomidin hydrochloride, 1mg/ml | |
| Flumazenil | Roche, Switzerland | 0.1 mg/ml | |
| Atipamezol | Orion Pharma, Finland | as Atipamezol hydrochloride, 5 mg/ml | |
| 10% povidone-iodine complex | Mundipharma, Austria | ||
| Dental cement | Heraeus Kulzer, Germany | 6603 7633 | |
| Physiological saline solution | Fresenius Kabi, Austria | 0.9% NaCl | |
| Phosphate buffered saline (PBS) | Sigma-Aldrich, Germany | P3813 | |
| Isofluorane | AbbVie, Austria | ||
| Lubricating eye drops | Thea Pharma, Austria | ||
| 70% EtOH | Merck, Germany | 1070172511 | Absolute ethanol was diluted in ddH2O for preparation of 70% v/v |
| 2.5% enrofloxacin | Bayer, Germany | Prophylactic antibiotics | |
| carprofen | Pfizer, USA | Painkillers, 50 mg/ml | |
| Tween-20 | Sigma-Aldrich, Germany | P9416 | |
| Pentobarbital | Richter Pharma, Austria | pentobarbital sodium, 400 mg/ml | |
| Interferon gamma | PeproTech, USA | 400-20 | |
| Tumor necrosis factor alfa | R&D Systems, USA | 510-RT-050/CF | |
| rMOG1-125 | own product at the Centre of Molecular Medicine, Karolinska Institute, Sweden | Recombinant rat myelin oligodendrocyte glycoprotein, amino acids 1-125 from the N-terminus, also commercially available: AnaSpec, AS-55152-500, USA | |
| Anti-MOG antibody | Ana Spec/Kaneka Corporation, Japan | AS-555157 | Standard from ELISA-Kit; Ana Spec/Kaneka |
| Incomplete Freund’s adjuvant | Sigma-Aldrich, Germany | F5506 | |
| Horse radish peroxidase conjugated anti-rat IgG secondary antibody | Ana Spec/Kaneka Corporation, Japan | AS-555157 | Secondary Antibody from ELISA-Kit; Ana Spec/Kaneka Corporation |
| Bovine serum albumin | Sigma-Aldrich, Germany | A9576 | |
| Peroxidase substrate solution | Vector Laboratories, USA | SK-45000 | |
| Stereotactic frame | David Kopf Instruments, USA | ||
| Catheters, MRI suitable | PlasticsOne, USA | 8IC315GPKXXC | |
| Dummy cannulas | PlasticsOne, USA | 8IC315DCNSPC | |
| Plastic screws, MRI suitable | PlasticsOne, USA | 8L080X093N01 | |
| Connector cannula | PlasticsOne, USA | 8IC313CXSPCC | |
| Screw driver with 2mm tip-size | |||
| Drill with flexible shaft extension | Proxxon, Germany | NO 28 472, NO 28 706, NO 28 620, | |
| Drill bit, round, 0.5 mm | Hager & Meisinger, Germany | REF310 104 001 001 009 | |
| Drill bit, twisted, 1.3 mm | Hager & Meisinger, Germany | REF350 104 417 364 013 | |
| Scalpel | Braun, Germany | BB510 | |
| Scalpel handle | Fine Science Tools, Germany | 91003-12 | |
| Cotton tip applicator | Henry Schein Medical, Austria | 900-3155 | |
| Surgical scissors | Fine Science Tools, Germany | 14101-14, 14088-10 | |
| Surgical forceps | Fine Science Tools, Germany | 11002-12, 11251-35 | |
| Bulldog clamps | Fine Science Tools, Germany | 18050-35 | |
| Homoeothermic blanket | TSE systems, Germany | ||
| Infrared Lamp | Beurer, Germany | 616.51 | |
| Dental curing light | Guilin Woodpecker Medical, China | ||
| Absorbable suture | Johnson & Johnson, Belgium | V792E | |
| Programmable syringe pump | World Precision Instruments, USA | AL-1000 | |
| Exam gloves | |||
| Surgical gown | |||
| Electric Shaver | Aesculap, Germany | GT420 | |
| Volatile anesthetic vaporizer | Rothacher Medical, Switzerland | CV 30-301-D | |
| Oxygen source for volatile anesthetic vaporizer | Air Liquide, Austria | 19,113 | |
| Volatile anesthesia chamber | Rothacher Medical, Switzerland | PS-0347 | |
| Anesthesia mask for rats | Rothacher Medical, Switzerland | PS-0307-A | |
| 1 ml syringe | Codan, Denmark | REF 62.1612 | |
| 26 Gauge needle for injection | Braun, Germany | 4657683 | |
| 20 Gauge needle for cytokine injection and immunization | Braun, Germany | 4657519 | |
| Luer lock tip glass syringes | Poulten & Graf, Germany | 7.140-37 | |
| 3 way stopcock | Becton Dickinson, Sweden | 394600 | |
| 96-well plate | Thermo Fisher Scientific, USA | 442404 | |
| Plate reader | Cole-Parmer, USA | EW-1396-00 | |
| 37°C incubator | Kendro, Germany | 50042301 | |
| Micropipettes | Gilson, USA | F167350 |
References
- Berkovich, R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics. 10 (1), 97-105 (2013).
- Kalincik, T. Multiple Sclerosis Relapses: Epidemiology, Outcomes and Management. A Systematic Review. Neuroepidemiology. 44 (4), 199-214 (2015).
- Feinstein, A., Freeman, J., Lo, A. C. Treatment of progressive multiple sclerosis: what works, what does not, and what is needed. The Lancet Neurology. 14 (2), 194-207 (2015).
- Montalban, X., et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. The New England Journal of Medicine. 376 (3), 209-220 (2017).
- Dyment, D. A., Ebers, G. C., Sadovnick, A. D. Genetics of multiple sclerosis. The Lancet Neurology. 3 (2), 104-110 (2004).
- Ascherio, A. Environmental factors in multiple sclerosis. Expert Review of Neurotherapeutics. 13, 3-9 (2013).
- Allen, I. V., McQuaid, S., Mirakhur, M., Nevin, G. Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurological Sciences. 22 (2), 141-144 (2001).
- Kutzelnigg, A., et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 128 (11), 2705-2712 (2005).
- Kutzelnigg, A., Lassmann, H. Cortical lesions and brain atrophy in MS. Journal of the Neurological Sciences. 233 (1-2), 55-59 (2005).
- Lucchinetti, C. F., et al. Inflammatory cortical demyelination in early multiple sclerosis. The New England Journal of Medicine. 365 (23), 2188-2197 (2011).
- Peterson, J. W., Bo, L., Mork, S., Chang, A., Trapp, B. D. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Annals of Neurology. 50 (3), 389-400 (2001).
- Kutzelnigg, A., Lassmann, H. Cortical demyelination in multiple sclerosis: a substrate for cognitive deficits. Journal of the Neurological Sciences. 245 (1-2), 123-126 (2006).
- Procaccini, C., De Rosa, V., Pucino, V., Formisano, L., Matarese, G. Animal models of Multiple Sclerosis. European Journal of Pharmacology. 759, 182-191 (2015).
- Kipp, M., Nyamoya, S., Hochstrasser, T., Amor, S. Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathology. 27 (2), 123-137 (2017).
- Lebar, R., Lubetzki, C., Vincent, C., Lombrail, P., Boutry, J. M. The M2 autoantigen of central nervous system myelin, a glycoprotein present in oligodendrocyte membrane. Clinical and Experimental Immunology. 66 (2), 423-434 (1986).
- Linington, C., Bradl, M., Lassmann, H., Brunner, C., Vass, K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. The American Journal of Pathology. 130 (3), 443-454 (1988).
- Panitch, H., Ciccone, C. Induction of recurrent experimental allergic encephalomyelitis with myelin basic protein. Annals of Neurology. 9 (5), 433-438 (1981).
- Tuohy, V. K., Sobel, R. A., Lees, M. B. Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. The Journal of Immunology. 140 (6), 1868-1873 (1988).
- Stromnes, I. M., Goverman, J. M. Active induction of experimental allergic encephalomyelitis. Nature Protocols. 1 (4), 1810-1819 (2006).
- Stromnes, I. M., Goverman, J. M. Passive induction of experimental allergic encephalomyelitis. Nature Protocols. 1 (4), 1952-1960 (2006).
- Mendel, I., Kerlero de Rosbo, N., Ben-Nun, A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and T cell receptor V beta expression of encephalitogenic T cells. European Journal of Immunology. 25 (7), 1951-1959 (1995).
- McRae, B. L., et al. Induction of active and adoptive relapsing experimental autoimmune encephalomyelitis (EAE) using an encephalitogenic epitope of proteolipid protein. Journal of Neuroimmunology. 38 (3), 229-240 (1992).
- Bebo, B. F., Vandenbark, A. A., Offner, H. Male SJL mice do not relapse after induction of EAE with PLP 139-151. Journal of Neuroscience Research. 45 (6), 680-689 (1996).
- Oliver, A. R., Lyon, G. M., Ruddle, N. H. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. The Journal of Immunology. 171 (1), 462-468 (2003).
- Scheld, M., et al. Neurodegeneration Triggers Peripheral Immune Cell Recruitment into the Forebrain. The Journal of Neuroscience. 36 (4), 1410-1415 (2016).
- Chanaday, N. L., Roth, G. A. Microglia and astrocyte activation in the frontal cortex of rats with experimental autoimmune encephalomyelitis. Neuroscience. 314, 160-169 (2016).
- Pomeroy, I. M., Matthews, P. M., Frank, J. A., Jordan, E. K., Esiri, M. M. Demyelinated neocortical lesions in marmoset autoimmune encephalomyelitis mimic those in multiple sclerosis. Brain. 128 (11), 2713-2721 (2005).
- Merkler, D., et al. Differential macrophage/microglia activation in neocortical EAE lesions in the marmoset monkey. Brain Pathology. 16 (2), 117-123 (2006).
- Storch, M. K., et al. Cortical demyelination can be modeled in specific rat models of autoimmune encephalomyelitis and is major histocompatibility complex (MHC) haplotype-related. Journal of Neuropathology and Experimental Neurology. 65 (12), 1137-1142 (2006).
- Ludwin, S. K. Central nervous system demyelination and remyelination in the mouse: an ultrastructural study of cuprizone toxicity. Laboratory Investigation. 39 (6), 597-612 (1978).
- Skripuletz, T., et al. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. The American Journal of Pathology. 172 (4), 1053-1061 (2008).
- Norkute, A., et al. Cuprizone treatment induces demyelination and astrocytosis in the mouse hippocampus. Journal of Neuroscience Research. 87 (6), 1343-1355 (2009).
- Acs, P., et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. 57 (8), 807-814 (2009).
- Pott, F., et al. Cuprizone effect on myelination, astrogliosis and microglia attraction in the mouse basal ganglia. Brain Research. 1305, 137-149 (2009).
- Jeffery, N. D., Blakemore, W. F. Remyelination of mouse spinal cord axons demyelinated by local injection of lysolecithin. Journal of Neurocytology. 24 (10), 775-781 (1995).
- Blakemore, W. F. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathology and Applied Neurobiology. 8 (5), 365-375 (1982).
- Mason, J. L., et al. Oligodendrocytes and progenitors become progressively depleted within chronically demyelinated lesions. The American Journal of Pathology. 164 (5), 1673-1682 (2004).
- Franco, P. G., Silvestroff, L., Soto, E. F., Pasquini, J. M. Thyroid hormones promote differentiation of oligodendrocyte progenitor cells and improve remyelination after cuprizone-induced demyelination. Experimental Neurology. 212 (2), 458-467 (2008).
- Lassmann, H., Bradl, M. Multiple sclerosis: experimental models and reality. Acta Neuropathologica. 133 (2), 223-244 (2017).
- Kerschensteiner, M., et al. Targeting experimental autoimmune encephalomyelitis lesions to a predetermined axonal tract system allows for refined behavioral testing in an animal model of multiple sclerosis. The American Journal of Pathology. 164 (4), 1455-1469 (2004).
- Merkler, D., Ernsting, T., Kerschensteiner, M., Bruck, W., Stadelmann, C. A new focal EAE model of cortical demyelination: multiple sclerosis-like lesions with rapid resolution of inflammation and extensive remyelination. Brain. 129, 1972-1983 (2006).
- Ucal, M., et al. Widespread cortical demyelination of both hemispheres can be induced by injection of pro-inflammatory cytokines via an implanted catheter in the cortex of MOG-immunized rats. Experimental Neurology. 294, 32-44 (2017).
- Gardner, C., et al. Cortical grey matter demyelination can be induced by elevated pro-inflammatory cytokines in the subarachnoid space of MOG-immunized rats. Brain. 136, 3596-3608 (2013).
- Minagar, A., et al. The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology. 80 (2), 210-219 (2013).
- Tsunoda, I., Kuang, L. Q., Theil, D. J., Fujinami, R. S. Antibody association with a novel model for primary progressive multiple sclerosis: induction of relapsing-remitting and progressive forms of EAE in H2s mouse strains. Brain Pathology. 10 (3), 402-418 (2000).
- Lifshitz, J., Witgen, B. M., Grady, M. S. Acute cognitive impairment after lateral fluid percussion brain injury recovers by 1 month: evaluation by conditioned fear response. Behavioural Brain Research. 177 (2), 347-357 (2007).
- Kabadi, S. V., Hilton, G. D., Stoica, B. A., Zapple, D. N., Faden, A. I. Fluid-percussion-induced traumatic brain injury model in rats. Nature Protocols. 5 (9), 1552-1563 (2010).
- Leonard, J. R., Grady, M. S., Lee, M. E., Paz, J. C., Westrum, L. E. Fluid percussion injury causes disruption of the septohippocampal pathway in the rat. Experimental Neurology. 143 (2), 177-187 (1997).
- Hare, G. M., et al. Severe hemodilutional anemia increases cerebral tissue injury following acute neurotrauma. Journal of Applied Physiology. 103 (3), 1021-1029 (2007).
- Abbott, N. J. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochemistry International. 45 (4), 545-552 (2004).
- Amor, S., et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. The Journal of Immunology. 153 (10), 4349-4356 (1994).
- Adzemovic, M. Z., Zeitelhofer, M., Hochmeister, S., Gustafsson, S. A., Jagodic, M. Efficacy of vitamin D in treating multiple sclerosis-like neuroinflammation depends on developmental stage. Experimental Neurology. , 39-48 (2013).

