A High-throughput, High-content, Liquid-based C. elegans Pathosystem
Summary
Here we describe a protocol that is an adaptable, whole host, high-content screening tool that can be utilized to study host-pathogen interactions and be used for drug discovery.
Abstract
The number of new drugs identified by traditional, in vitro screens has waned, reducing the success of this approach in the search for new weapons to combat multiple drug resistance. This has led to the conclusion that researchers do not only need to find new drugs, but also need to develop new ways of finding them. Amongst the most promising candidate methods are whole-organism, in vivo assays that use high-throughput, phenotypic readouts and hosts that range from Caenorhabditis elegans to Danio rerio. These hosts have several powerful advantages, including dramatic reductions in false positive hits, as compounds that are toxic to the host and/or biounavailable are typically dropped in the initial screen, prior to costly follow up.
Here we show how our assay has been used to interrogate host variation in the well-documented C. elegans—Pseudomonas aeruginosa liquid killing pathosystem. We also demonstrate several extensions of this well-worked out technique. For example, we are able to carry out high-throughput genetic screens using RNAi in 24- or 96-well plate formats to query host factors in this host-pathogen interaction. Using this assay, whole genome screens can be completed in only a few months, which can dramatically simplify the task of identifying drug targets, potentially without the need for laborious biochemical purification approaches.
We also report here a variation of our method that substitutes the gram-positive bacterium Enterococcus faecalis for the gram-negative pathogen P. aeruginosa. Much as is the case for P. aeruginosa, killing by E. faecalis is time-dependent. Unlike previous C. elegans—E. faecalis assays, our assay for E. faecalis does not require preinfection, improving its safety profile and reducing the chances of contaminating liquid-handling equipment. The assay is highly robust, showing ~95% death rates 96 h post infection.
Introduction
The identification and development of effective, broad-spectrum antibiotics, now almost a century ago, led to a watershed moment in public health where there was a wide-spread belief that infectious disease would be a scourge of the past. Within a few short decades, this optimism began to wane, as pathogen after pathogen developed resistance mechanisms that limited these once miraculous treatments. For some time, the arms race between drug discovery efforts and the pathogens seemed balanced. However, the misuse of antimicrobials has recently culminated in the emergence of pan-drug resistant strains of Klebsiella pneumoniae, Acinetobacter baumanii, Serratia marcescens, and P. aeruginosa1,2,3,4.
P. aeruginosa is an opportunistic, gram negative, multi-host pathogen that is a severe threat to patients with severe burns, those who are immunocompromised, or have cystic fibrosis. It is also increasingly identified as a causative agent in severe nosocomial infections, particularly due to its ongoing acquisition of antimicrobial resistance. To begin to address this threat, we have used the well-documented C. elegans–P. aeruginosa infection system5. Our lab has leveraged this system to develop a liquid-based, high-throughput, high-content screening platform to identify novel compounds that limit the ability of the pathogen to kill the host6. Intriguingly, these compounds seem to belong to at least three general categories, including antimicrobials7 and virulence inhibitors8. Other high-content drug discovery assays in C. elegans have been reported for Mycobacterium tuberculosum, Chlamydia trachomatis, Yersinia pestis, Listeria monocytogenes, Francisella tularensis, Staphylococcus aureus, Candida albicans, and Enterococcus faecalis, among others9,10,11,12,13,14,15,16. These types of assays have several well-recognized advantages, such as limiting false positive hits that may be toxic to both the host and the pathogen, increased likelihood of bioavailability compared to a chemical screen, and the ability to identify hits beyond simply limiting microbial growth, such as anti-virulents, immune stimulatory molecules, or compounds that otherwise tilt the balance of the host-pathogen interaction in favor of the former. Additionally, the compounds discovered in these screens are often effective in mammalian hosts.
It is worth noting that at least two other assays17,18 are available to carry out high-throughput screens in C. elegans in liquid. However, each of these assays is a modification that allows the prototypical intestinal-colonization assay, known as slow-killing, to be performed in liquid, increasing throughput and allowing compounds to be more readily screened. Careful characterization has conclusively demonstrated that the mechanisms of bacterial virulence are different between these assays and our liquid-based screen7. Since both types of virulence are observed in mammalian systems, it is important to consider which virulence determinant is most relevant for the experimenter's interests prior to assay selection.
Here we demonstrate an optimized version of the liquid-based C. elegans-P. aeruginosa assay. We also report the adaptation of our liquid-based assay method to accommodate the gram-positive bacterial pathogen Enterococcus faecalis. Like P. aeruginosa, E. faecalis is increasingly identified as a serious nosocomial threat with a growing armament of antimicrobial resistance pathways1. Although a previous method for high-throughput screening of E. faecalis exists14, it requires preinfection with the pathogen, which complicates the procedure and increases the likelihood of contaminating equipment like the COPAS FlowSort. Our protocol eliminates the need for pre-infection, improving the safety profile. Finally, we report a means by which either of these assays can be combined with feeding RNAi, allowing the user to search for host factors that play a role in the establishment of, or resistance to, infection.
Protocol
Caution: P. aeruginosa and E. faecalis are Biosafety Level 2 pathogens, and proper safety precautions must be taken to prevent accidental infection and to minimize contamination of surfaces. All media and materials that come into contact with pathogens must be sterilized and/or discarded. Further guidelines are available from the CDC publication Biosafety in Microbiological and Biomedical Laboratories (BMBL), 5th edition.
1. Preparation and Maintenance of P. aeruginosa
- Streak P. aeruginosa from a frozen stock onto an LB (Lysogeny Broth) agar plate. Incubate 16 to 24 h at 37 °C
Note: Perform P. aeruginosa experiments inside of a BSL-2 biological safety cabinet to ensure sterility. Use appropriate safety precautions to minimize the chance of pathogenic infection or contamination. - Transfer this plate to 4 °C. This plate may be retained at 4 °C and used to inoculate cultures for up to one week. After one week, decontaminate and discard the plate and make a new LB streak plate.
Note: P. aeruginosa virulence decreases after prolonged storage. - Two days prior to setting up assay plates, inoculate 3 – 5 mL of sterile LB broth with a single colony of P. aeruginosa from the plate made in 1.1. Incubate at 37 °C for 12 to 16 h.
Note: Do not incubate longer than 16 h. In rich liquid cultures, P. aeruginosa PA14 begins to lyse. - Prepare sterile 10 cm plates with Slow Kill media (3 g NaCl, 3.5 g peptone, 1 mM CaCl2, 1 mM MgSO4, 25 mL of phosphate buffer (amount per liter: 132 mL of K2HPO4 (1M) and 868 mL of KH2PO4 (1M)) and 18 g agar/L).
Note: Prepare plates ahead of time. They can be stored for up to 3 weeks in an airtight container at 4 °C. - Seed each 10 cm Slow Kill plate (SK plate) with 350 μL of P. aeruginosa from fresh overnight LB culture. Using a sterile bacterial spreader, spread bacteria evenly across surface of media and allow to dry in a BSL-2 biological safety cabinet.
- Incubate plates at 37 °C for 24 h.
2. Preparation of RNAi Bacteria
- Streak out or pin-transfer desired strains of RNAi-containing bacteria onto LB agar plates containing appropriate antibiotics (carbenicillin at 100 µg/mL and tetracycline at 15 µg/mL for Ahringer and Vidal libraries) from a frozen stock.
Note: When working with nonpathogenic bacteria, use either a BSL-2 A/B biological safety cabinet or a BSL-1 laminar flow hood to ensure sterility of the cultures and to minimize the chance of cross-contamination of the library. - Incubate plates for 24 h at 37 °C.
- Store the plates at 4 °C for up to two weeks.
- After two weeks, discard the plates and replace them with freshly made plates.
- Test multiple RNAi strains in parallel by individually inoculating a single colony from each clone into 4 mL of carbenicillin-supplemented LB in a single well of a 24-well deep-well plate or into a sterile test tube.
Note: Tetracycline has been reported to reduce the efficiency of RNAi. Do not use it in this media. - Place 24-well deep well plates into a shaking incubator optimized for multiwell plates and incubate at 37 °C for 16 h while shaking at 950 rpm.
Note: It is hard to obtain uniform bacterial growth in multiwell plates using a conventional shaking incubator. The shaking incubator needs to be able to shake at a minimum of 750 rpm in order for the cultures to grow properly. In the absence of a specialized shaker, it is possible to grow each culture in an individual tube. Cultures can be transferred into the 24-well plate for centrifugation afterward, if desired. - Collect bacteria by centrifuging for 5 minutes at 2,000 x g.
- Decant supernatant by inverting the plate and shaking vigorously. Resuspend RNAi bacteria in 100 µL of S Basal (5.85 g NaCl, 1 g K2HPO4, 6 g KH2PO4 dissolved into 1 L of ultrapure water and sterilized by autoclave).
- Pipette resuspended bacteria into an appropriate number of wells of a multiwell NGM plate (Nematode Growth Media, 3 g NaCl, 2.5 g peptone, 1 mM CaCl2, 1 mM MgSO4, 25 mL of phosphate buffer (amount per liter: 132 mL of K2HPO4 (1M) and 868 mL of KH2PO4 (1M)), and 18 g agar per liter of water) supplemented with IPTG (1 mM final concentration) and carbenicillin (100 μg/mL final concentration).
- Use 3.5 mL of NGM media per well of 6-well plate, 1 mL per well of a 24-well plate, or 150 µL per well of 96-well plate.
- Use 100 μL/well of bacteria for 6-well plate; 50 μL/well for 24-well; or 20 μL/well for 96-well plate. Allow to dry.
Note: Drying is normally performed in a sterile flow hood to promote rapid, uniform drying. Uniform drying is very important. Avoid over-drying, as cracks in the agar promote burrowing of worms. This leads to worm losses and potential clogging of liquid-handling systems.
- Use the prepared plates immediately or store them at 4 °C for up to two weeks for later use.
Note: To prevent plates from drying out, they can be sealed with plastic paraffin film or placed in a tightly-sealed container with damp paper towels.
3. Maintenance and Preparation of C. elegans
Note: Before initiating experiments, generate a synchronized population of gravid, adult hermaphroditic worms as follows.
- Wash gravid adults (i.e., with multiple embryos visible within the gonad) from 4 – 6 10-cm NGM plates into a 15 mL conical tube by adding 4 mL of S Basal to plate, swirling gently, and then pouring into a 15 mL conical tube.
- Pellet worms via centrifugation at 1,000 x g for 30 seconds.
- Aspirate supernatant, taking care not to disrupt the worm pellet.
- Add 3.5 mL of worm bleach solution (final concentration 0.5M NaOH, 1% sodium hypochlorite, in sterile water) to the worm pellet, and mix by inverting for 1 minute.
- Briefly vortex and centrifuge at 1,000 x g for 30 seconds.
- Aspirate or decant the supernatant, taking care not to disturb the worm pellet.
- Add 3.5 mL of fresh worm bleach solution to the worm pellet.
- Vigorously mix worms in bleach solution. Periodically (approximately every ten seconds) visually inspect worms under a dissecting microscope. Watch for worm cuticles to break, releasing eggs. Once most of the adult worms have been dissolved (~95%), dilute bleach to 15 mL with S Basal to stop digestion.
Note: Egg shells are more resistant to bleach than adult tissues, but they can be dissolved during prolonged exposure. To avoid egg dissolution, do not expose eggs to worm bleach solution for longer than 7 minutes. If egg shells are excessively damaged, the embryos will die. - Pellet embryos at 1,000 x g for 30 seconds and aspirate or decant the supernatant without removing the pellet of embryos.
- Resuspend worms in S basal to a final volume of 15 mL to dilute remaining bleach solution.
- Repeat the washing steps (3.9 - 3.10) three more times for a total of 4 consecutive washes.
- After the fourth wash, aspirate supernatant and add up to 5 or 6 mL of sterile S Basal into the 15 mL conical tube. The goal is to resuspend embryos to a concentration of ~50 worms per µL.
Note: The amount of S Basal depends upon the size of the egg pellet; the larger the pellet, the more S Basal is necessary. - Incubate the conical tube overnight on a table-top rotator at room temperature or 48 h at 15 °C. Larvae will hatch, but larval development will arrest at the L1 stage (L1 diapause) due to nutrient deprivation.
- Examine the embryos under a dissecting microscope to verify that most of them have hatched and arrested at the L1 stage of development.
- Determine the worm count by taking 20 µL of worm prep and diluting it with 80 µL of S Basal. Pipette 10 µL of diluted worm solution directly onto a blank NGM plate. Repeat 2 more times.
- Count the larvae in each spot and determine the average number. Divide this average by 2 to obtain an approximate count of worms per µL in the worm prep. Worm preps can then be used for propagation plates or for experiments.
Note: After 4 – 5 days, starved larvae will begin to die; discard the prep at this point.
- Count the larvae in each spot and determine the average number. Divide this average by 2 to obtain an approximate count of worms per µL in the worm prep. Worm preps can then be used for propagation plates or for experiments.
- For propagating the strain, pipette an appropriate number of L1 worms (5,000 – 6,000) onto 3 – 4 10-cm NGM plates spotted with concentrated OP50 E. coli – “superfood” (E. coli OP50 spotted at 25X concentration (i.e., 1 L of overnight OP50 culture grown in LB yields 40 mL of 25X OP50 superfood); 2 mL of this concentrated bacterial suspension are spotted onto each 10 cm NGM plate).
- To prepare plates for experiments, do one of the following (for “Basic Protocol” use 3.17.1 setup, for the “RNAi screen set up” use steps 3.17.2 – 3.17.4 as appropriate):
- Pipette ~ 5,000 worms/10 cm plate seeded with RNAi or OP50 superfood.
- Pipette ~ 500 worms/well in a 6-well plate seeded with RNAi.
- Pipette ~300 worms/well in a 24-well plate seeded with RNAi.
- Pipette ~100 worms/well in a 96-well plate seeded with RNAi.
Note: For instructions on how RNAi was seeded please refer to steps 2.7 – 2.9 in Preparation of RNAi Bacteria.
- If a fertile strain of worms is being used, incubate worms at 25 °C for ~44 – 48 h. Use these worms as quickly as possible after the population has reached the appropriate age. This minimizes the impact of egg hatching within the worms during the assay.
- If the strain being used is temperature-sterile (e.g., fer-15(b26); fem-1(hc17) or glp-4(bn2)), incubate worms at 15 °C for ~16 h and then transfer to 25 °C for 44 h to complete development and prevent embryogenesis.
4. Liquid Killing Assay Setup (Basic Protocol)
Note: This is a protocol for one bacterial strain and one source of worms.
- Using a cell scraper, remove the P. aeruginosa from an SK plate and resuspend in ~ 5 mL of S Basal. Scale up if needed. Measure the optical density of the bacterial suspension using a spectrophotometer (OD600)
- Prepare 24 mL of diluted stock of P. aeruginosa in S Basal at OD600 ≈ 0.09 (3X final concentration). See 4.6.2 for final content per well, and scale volume of bacterial dilution accordingly.
- Add 21 mL of liquid Slow Kill media (3 g of NaCl, 3.5 g of peptone/L supplemented with CaCl2 and MgSO4 to a final concentration of 1 mM). Using a multichannel pipette, transfer 45 µL of bacteria and media to each well of a 384-well plate.
- Wash worms from their source (i.e., Step 3.17.1) into a 50 mL conical tube and allow worms to settle under gravitational force. Aspirate supernatant to 5 mL. Resuspend in a total of 50 mL S basal.
- Repeat step 4.4 twice.
- Using a worm sorter, sort approximately 22 worms into each well of the 384-well plate.
Note: The setup drops each worm in ~1.1 µL of liquid. 22 worms will amount to 25 µL, bringing total assay volume to 70 µL/well.- The final composition of each well consists of 70 µL. 45 µL of this volume is added as bacterial medium (0.03 OD600 bacteria in 24 µL S Basal, and 21 µL SK supplemented with CaCl2 and MgSO4). The other 25 µL is added with the 22 worms.
- If the sorter used here (see Table of Materials) is unavailable, worms can be diluted to a final concentration of 1 worm/µL in S Basal and pipetted 25 µL/well using a multichannel pipette. However, results may exhibit increased variability. Alternatively, it is possible to use a peristaltic liquid dispenser, as described by Leung and colleagues19.
- After worms have been sorted into the 384-well plate, seal the plate with a gas-permeable film and incubate plates at 25 °C for 24 – 48 h.
- At the desired time, use a microplate washer to wash the 384-well plate with S Basal a total of 5 times.
- After the second wash, aspirate most of the media, leaving ~20 µL. Vigorously shake plates using a microplate vortexer for at least 30 seconds to loosen any debris from the bottom of the wells).
- After the final wash, aspirate supernatant down to 20 µL. Add 50 µL of 0.98 µM nucleic acid stain (see Table of Materials)/well of the 384-well plate, for a final concentration of 0.7 µM.
- Incubate at room temperature for 12 – 16 h. This dye will only stain dead worms. After the desired incubation period, wash plates using the microplate washer to remove any excess stain (a minimum of 3 washes).
- For data acquisition, use a spectrophotometer or an automated microscope to image both transmitted light and fluorescence (531 nm excitation and 593 emission).
- Use a low magnification objective to allow an image of the whole well to be captured.
- Alternatively, use flow vermimetry. This technique uses a large object flow cytometer as a worm fluorimeter, measuring the size and fluorescence of whole organism (i.e., C. elegans) in a fashion that is strongly similar to flow cytometry.
- Use automated image analysis software (such as CellProfiler, a free image analysis studio based on MatLab http://cellprofiler.org/) to calculate the fluorescent and total worm areas per well in an unbiased fashion.
Note: The quotient of these numbers represents the fraction death for each well. If one well had 7 stained worms out of 20 total, then the fraction death comprises 0.35 or 35%.- Prior to first use, the automated image processing pipeline must be validated by manual scoring. This is done by comparing results from the automated pipeline to scores obtained by visually inspecting images and recording fractions of dead worms for each of them. The validation process ensures that the parameters for the automated imaging processing are accurate and represent the data correctly.
5. Adaptation for Screening Multiple C. elegans Strains or Knockdowns (RNAi Screen Setup)
Note: This is an RNAi screen described for a 24-well plate setup.
- Add 1 mL of S Basal per well of a 24-well plate (see step 3.17.3). Gently agitate worms by shaking the plate, then transfer worms to an empty, sterile 24 deep-well plate. Allow worms to settle gravitationally (~5 minutes).
- Aspirate supernatant, leaving approximately 1 mL. Add 7 mL of S Basal to each well.
- Repeat steps 5.1 – 5.2 a minimum of 2 more times. After final wash, aspirate supernatant, leaving approximately 400 µL.
- Using the Resampler function of the worm sorter, sort 22 worms from the 24-well plate worm suspension into each well of a 384-well plate (each well of a 24-well plate yields 8 – 12 wells of a 384-well plate).
- To avoid starvation, pipette bacteria into 384-well plates (either a strain that serves solely as food, such as OP50, or a pathogenic strain) prior to sorting.
Note: Pathogens can be added by following steps 2.3 – 2.5 or 6.4 – 6.5, and food source bacteria can be diluted and added by following the same scheme as for P. aeruginosa.
- To avoid starvation, pipette bacteria into 384-well plates (either a strain that serves solely as food, such as OP50, or a pathogenic strain) prior to sorting.
- After worms have been sorted into the 384-well plate, add small molecules or other experiment-specific materials.
6. Adaptation for E. faecalis
Note: Only the differences from P. aeruginosa assay are described.
- Prepare a fresh source of E. faecalis by streaking bacteria from a frozen stock onto a BHI (Brain Heart Infusion) agar plate and incubating for 16 to 24 h at 37 °C.
Note: Perform E. faecalis experiments inside of a BSL-2 biological safety cabinet to ensure sterility. Use appropriate safety precautions to minimize the chance of pathogenic infection or contamination. - Plates can be stored at 4 °C for up to one week. After this period, replace with a fresh plate.
- The night before sorting worms, inoculate 3 – 5 mL sterile BHI with a single colony of E. faecalis. Incubate 12 – 16 h at 37 °C with agitation.
- Add bacteria to the wells as for P. aeruginosa (3x bacteria in S Basal, OD600≈0.09).
Note: For E. faecalis, the final well composition is: E. faecalis (OD600≈0.03), BHI = 10% v/v, with the remaining volume comprised of S basal. Remember that 25 µL of S basal will be delivered with worms.
Note: E. faecalis produces thick biofilms that absorb the fluorescent stain, causing blurred images. Extensive washing may be insufficient to remove this biofilm. In this case, worms can be transferred to an empty plate using a multichannel pipette. To facilitate transfer, Tween 20 can be added to S Basal to a final concentration of 0.1% (v/v) Tween 20 in S Basal. This aids in removing the worms from the biofilm.
Representative Results
Important parameters for assay performance
A proper understanding of the biology underlying this assay is necessary for troubleshooting and optimizing the assay. To that end, we refer first to several key papers elucidating the mechanisms of pathogenesis of P. aeruginosa-mediated killing in liquid7,20. Provided that the steps outlined above are followed (see Figure 1 for a schematic of the assay protocol) a time-dependent killing of C. elegans will be observed only in the presence of the pathogen (Figure 2A). In contrast, in the absence of key nutritional supplements (e.g., if the peptone is left out of the media, and only S Basal is added), little to no killing will be observed (Figure 2B). Interestingly, the two-step incubation of P. aeruginosa (for 24 h at 37 °C, then 24 h at 25 °C) which is critical for conventional slow-killing assays, and was originally implemented in Liquid Killing21, is dispensable in this assay (Figure 2C). While it is possible to add P. aeruginosa straight from overnight LB culture, doing so significantly changes lethality kinetics and is not recommended.
Understanding assay biology also permits simplification of the assay, if appropriate and desirable. For example, the most important driver of host killing in this assay is the siderophore pyoverdine, and host toxicity caused by pyoverdine is contingent upon its ability to bind iron7. As such, the assay can be simplified by substituting pyoverdine-rich filtrate, purified pyoverdine, or even some synthetic iron-chelating chemicals (e.g., 1,10-phenanthroline) for live bacteria, as the transcriptional response of C. elegans to these treatments is very similar (Figure 3)22.
Several different lines of evidence speak to the robustness of the experimental setup. First, the setup tolerates a wide range of initial bacterial concentrations. Concentrations as low as OD600 = 0.0025 (approximately 10-fold lower than recommended) still exhibit time- and concentration-dependent killing, although the timing does shift (Figure 4A). Second, the reproducibility of the assay is such that as few as 4 wells is frequently sufficient to obtain statistically significant data (Figure 4B).
There are several changes that we do not recommend. For example, using initial bacteria inocula with concentrations much higher than those listed in the protocol; doing so results in thick, biofilm-like material that is difficult or impossible to effectively wash away, which complicates scoring lethality. Furthermore, high-concentration bacterial inocula also can compete for oxygen and trigger non-specific host killing7.
Another important note is to limit the period of time between stopping the assay and imaging the dead worms. Note that this time frame must include staining, so it is critical to be efficient. After death, the biological material within worms begins to extrude and/or be consumed by bacteria. Within a matter of 24 – 32 h, only the cuticle remains. The cuticle stains very poorly and is very light, making it easy to lose during washes. It is important to note that strains or strain/RNAi conditions with very different kinetics of killing can be complicated by this phenomenon (i.e., some worms may still be alive while others have already lost their content and are impossible to image). Figure 4A shows this phenomenon, as worms at the left side of the plate, inoculated with very low initial bacterial concentrations, are still largely alive while worms on the right side of the plate, which were exposed to much higher concentrations of bacteria, have been washed away.
A very important determinant of assay success is the method used to collect and analyze the data. In our lab, we have used at least three methods for collecting data from these assays: spectrophotometry, automated microscopy, and flow vermimetry (i.e., the adaptation of flow cytometry techniques to C. elegans; literally, the measurement of worms in a flowing solution). The first of these methods uses a spectophotometer to read the fluorescence of the dye or reporter of the worms in question. This method has the advantage of using a fairly ubiquitous piece of equipment (a standard spectrophotometer with a microplate reader) and is the fastest method to acquire data. However, it lacks the informational content available from automated microscopy and the statistical power of flow vermimetry.
Automated microscopy is another viable option. A number of microplate imaging systems are currently on the market, ranging in prices that are affordable to a single investigator (e.g., a Bio-Tek Cytation5) to larger, more expensive and higher-quality machines that are more commonly found in core facilities (e.g., a Molecular Devices ImageXpress Microscope). In practice, most of these solutions are amenable for scoring most screens and assays. Most automated imaging platforms can also be coupled to downstream software for image processing (e.g., MetaMorph, ImageJ, Gene5, or Cell Profiler) to further the yield of information. Examples of the utility of processing are shown in Figure 5 and Figure 6. When the signal-to-noise ratio is high (as in Figure 5),analysis is simple and discriminating between positive and negative conditions is trivial. In these cases, even weak hits can be readily identified. Many assays, however, have a weaker signal-to-noise ratio. For example, treatment of PINK-1::GFP22 worms (with constitutive mCherry expression in their pharynges) with 1,10-phenathroline increases the level of PINK-1::GFP. For data analysis, the inducible GFP reporter is normalized to the constitutive mCherry signal (Figure 6). In this case, the reporter has weaker expression and/or is not activated in the majority of the worms. Therefore, implementing additional image-processing tools, like Cell Profiler, that can reduce background and amplify signal may be advantageous.
Finally, if a COPAS FlowPilot is available, it can be used to acquire data via flow vermimetry. Much like the more familiar concept of flow cytometry, this instrument can be used to measure the fluorescence of C. elegans in at least two or three channels at a time. This method is very amenable to the acquisition of data with high statistical significance, but the throughput is very much diminished compared to automated microscopy. The most significant advantage of flow vermimetry is in the ability to analyze whole worm populations as a single group for a replicate, rather than splitting them into multiple wells and analyzing the average of the wells. Analyzing large groups in this fashion dramatically improves the statistical power and allows the detection of even small effects.
Modifications of the Liquid Killing Assay
Recent developments of the assay have extended its usefulness by including assaying activation of fluorescent reporters (Figure 6), using medium- to high-throughput RNAi techniques (Figure 3), and even the substitution of the original pathogen.
The most obvious reason to use RNAi set up is to test for host defense pathways against P. aeruginosa (or other pathogens). Examples of RNAi knockdown of genes relevant for survival in the assay are shown in Figure 3. Consistent with previous observations20,23,24,25, daf-2(RNAi) enhanced C. elegans survival during exposure to P. aeruginosa, while daf-16(RNAi), zip-2(RNAi) and cebp-1(RNAi) shortened it. As noted, RNAi is most simply included in the assay growing worms on multiwell plates where each well contains a different RNAi clone. Due to the high viscosity of the agar and small volumes involved, it is difficult to achieve uniform, bubble-free filling of 96-well plates without specialized equipment. In addition, drying the bacterial strains carrying the RNAi can also be an issue, as the outer wells dry faster than inner wells, leading to non-uniformity and significant potential for agar cracking. As noted above, this encourages worms to burrow, reducing the number of animals available for screening and risks clogging liquid-handling machinery. For both of these reasons, 24-well plates are easier to work with than 96-well plates. Although this reduces assay throughput, it improves reliability, and is suggested, particularly for initial screens.
Finally, the assay has been modified the assay to replace P. aeruginosa with E. faecalis. (In principle, substitution with other bacterial species should not provide undue difficulties, provided that appropriate culturing and exposure conditions are identified.) The most important factor to consider is the media requirements of the pathogen, both for growth and development and for the induction of virulence. For example, the Gram positive, opportunistic pathogen E. faecalis requires nutrient-rich media (like BHI) and incubation at higher temperatures compared to P. aeruginosa14,26. By taking these factors into account, adaptation of the the assay to use E. faecalis was straightforward. As noted for P. aeruginosa, we saw time-dependent killing (Figure 7). Interestingly, the timing of death was similar to previously published assays that used pre-infection on agar plates26, despite the absence of this step, which may suggest that the mechanisms involved in pathogenesis vary.
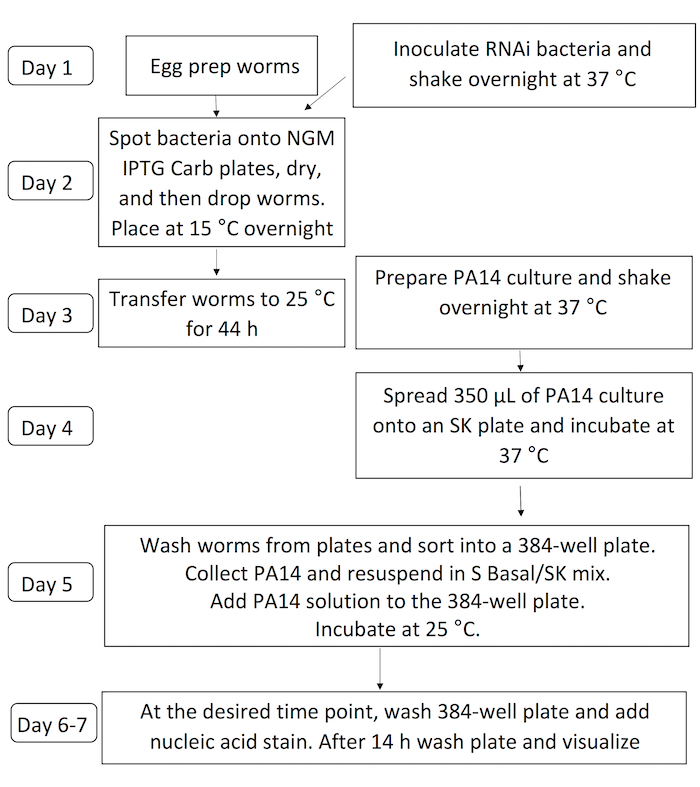
Figure 1: Scheme for liquid-based C. elegans – P. aeruginosa infection assay. Steps involving nematode propagation and preparation are at left side, bacterial preparation are at right. Steps involving both are centered. Please click here to view a larger version of this figure.
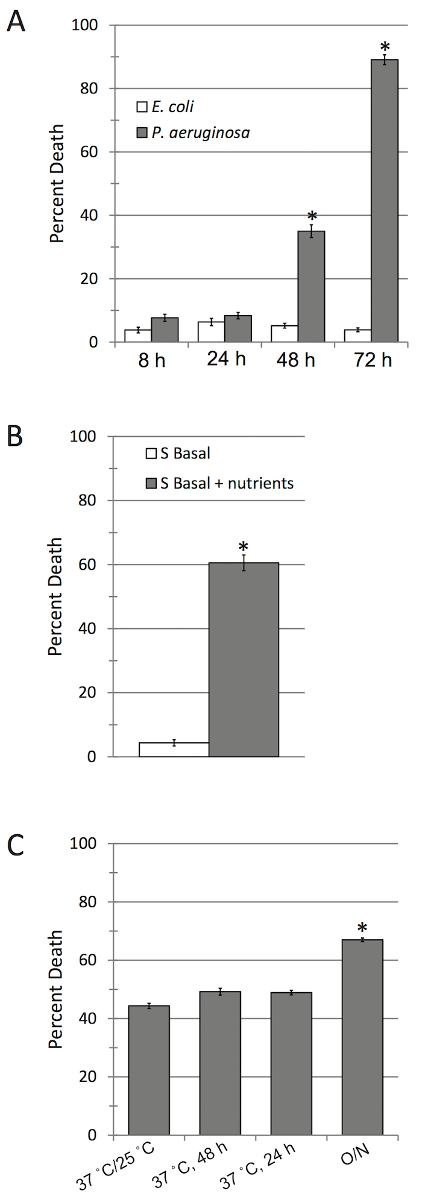
Figure 2: Killing of C. elegans by P. aeruginosa is specific and requires proper bacterial nutrition. (A) C. elegans death in the presence of E. coli and P. aeruginosa. (B) C. elegans death in the presence of P. aeruginosa with (right) and without (left) Slow Kill media added to mix (i.e., S basal only). S Basal is a minimal media and does not contain the nutrients required for bacterial growth and precludes the production of pyoverdine. (C) C. elegans death in the presence of differently prepared P. aeruginosa. *p <0.01, based on Student's t-test. Error bars represent S.E.M. 10 wells were used per biological replicate per condition. Please click here to view a larger version of this figure.
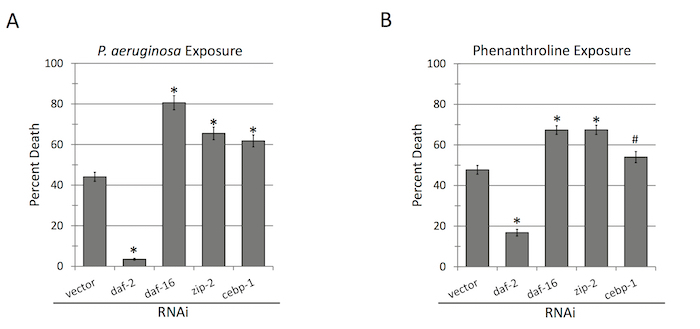
Figure 3: C. elegans resistance to P. aeruginosa depends on host immune pathways. A panel of selected RNAi knockdowns was treated with either P. aeruginosa (A) or 1,10-phenanthroline (a chemical chelator that mimics exposure to P. aeruginosa in liquid) (B). *p <0.01, #p <0.05, based on Student's t-test. Error bars represent S.E.M. 10 wells were used per biological replicate per condition. Please click here to view a larger version of this figure.
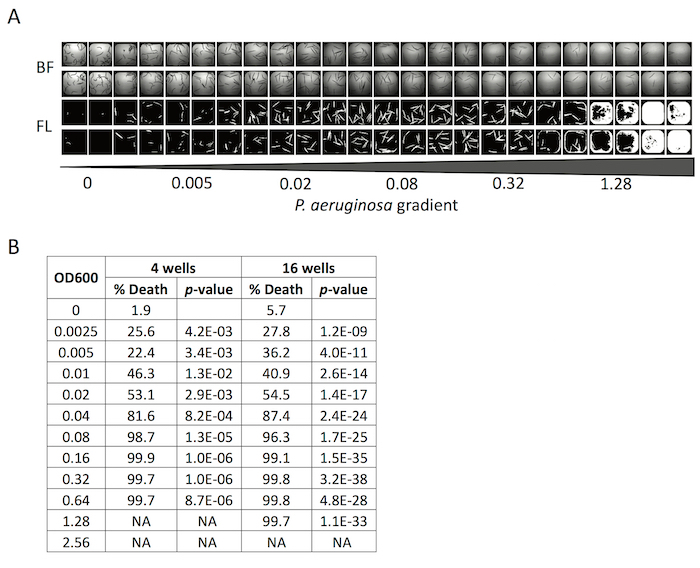
Figure 4: C. elegans killing by P. aeruginosa is robust and depends on bacterial concentration. (A-B) Representative images (A) and quantification (B) of C. elegans death after exposure to varying concentrations of P. aeruginosa for 72 h. p-values were calculated based on Student's t-test. Scale bar in (A) is 1 mm. 16 wells were used per biological replicate per condition. BF: Bright Field, FL: Fluorescence. Please click here to view a larger version of this figure.
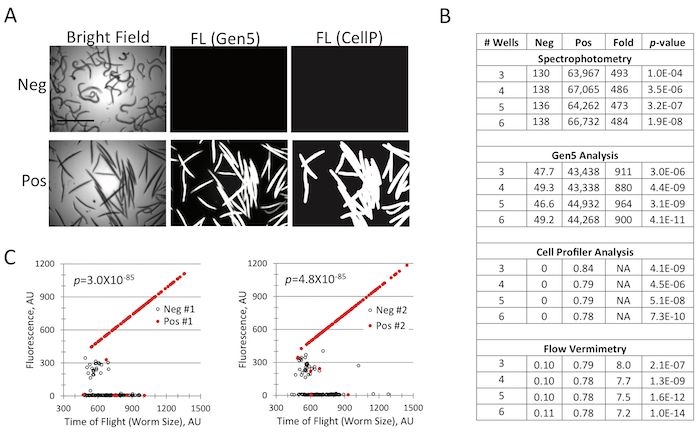
Figure 5: Data acquisition and image processing of an assay with a strong signal. (A) Representative images of live (negative control) and dead (positive control) C. elegans. (B) Statistical analysis of negative and positive controls based on data acquisition method, processing mode, and number of wells analyzed. (C) Fluorescence was analyzed for worms from four individual wells of a 96-well plate (two positive and two negative control wells). Each well contained ~100 worms. Each dot on the graph represents a single worm, showing the time-of-flight (which correlates with but, due to the coiling of living worms, imperfectly represents size) and the measured fluorescence for worms that are stained with Sytox Orange. Positive control worms were pre-killed by heat exposure. Due to changes in tension and shape of dead worms (as can be seen in (A), dead worms appear more rod-like and have few body bends) compared to their living counterparts, dead worms have a longer TOF. p-values were calculated based on Student's t-test. Scale bar in (A) is 1 mm. Please click here to view a larger version of this figure.
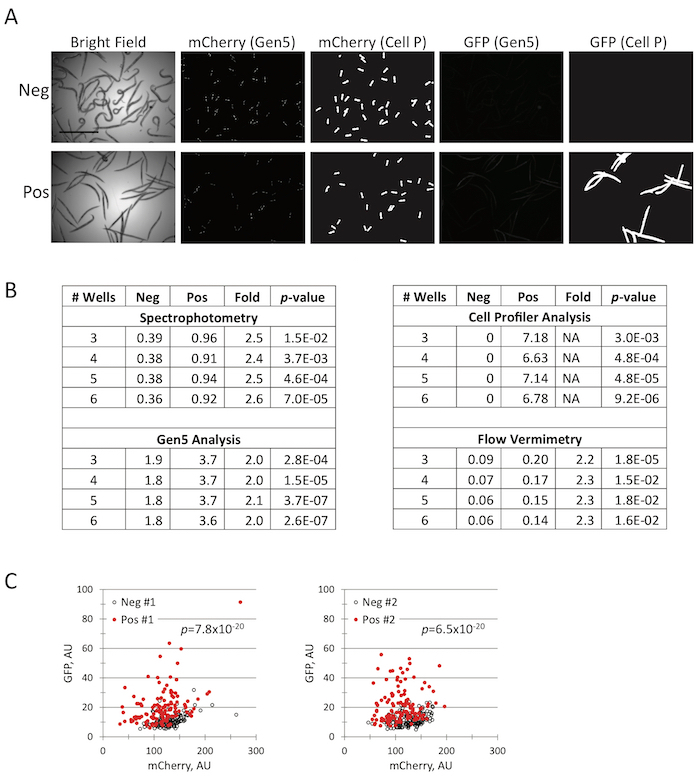
Figure 6: Data acquisition and image processing of an assay with a moderate signal. (A) Representative images of C. elegans carrying an inducible PINK-1::GFP reporter and an mCherry constitutive marker (to normalize expression of genes from the extrachromosomal array). C. elegans exposed to DMSO (negative control) or 1,10-phenanthroline (positive control). (B) Statistical analysis of negative and positive controls based on data acquisition method, processing mode, and number of wells analyzed. (C) Four individual wells of a 96-well plate (two positive and two negative control wells) were analyzed using flow vermimetry. p-values were calculated based on Student's t-test. Scale bar in (A) is 1 mm. Please click here to view a larger version of this figure.
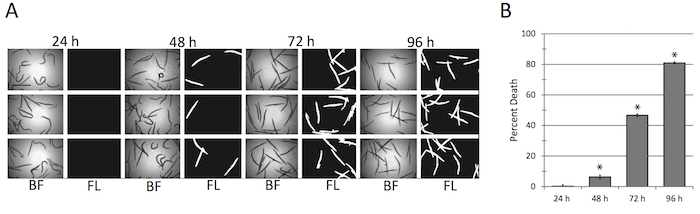
Figure 7: C. elegans killing by E. faecalis is robust and time dependent. (A-B) Representative images (A) and quantification (B) of C. elegans death after exposure to E. faecalis. *p <0.01, based on Student's t-test. 10 wells were used per biological replicate per condition. Error bars represent S.E.M. Scale bar in (A) is 1 mm. Please click here to view a larger version of this figure.
Discussion
This assay (or similar assays where other pathogens are substituted for P. aeruginosa or E. faecalis) is useful for a variety of purposes, including drug discovery. It is also useful for addressing fundamental biological questions, such as identifying virulence factors, the elucidation of host defense pathways, and determining the regulatory machinery involved in the host-pathogen interaction.
Although the P. aeruginosa Liquid Killing assay is robust, there are several points where careful attention should be paid to minimize the chance of failure. First, as noted, the bacterial source plate for P. aeruginosa inoculations must remain fresh, lest the bacteria lose virulence, despite overnight growth in LB. Second, it is key to make sure that calcium and magnesium are added to P. aeruginosa killing assays. Without it, virulence will be significantly compromised. Finally, it is worth noting that worms reared on HT115 (for RNAi-based assays) will die significantly later than worms reared on OP50 (N. Kirienko, personal communication). For this reason, if switching to RNAi-based studies, it is important to empirically determine the best time point for the assay.
For killing assays with either P. aeruginosa or E. faecalis, it is crucial to ensure that there is adequate food for the development of the worms from the L1 stage until they are ready to be used in the assay. Starvation, even in the short-term, is known to activate a number of host defense pathways, such as the DAF-2/DAF-16 insulin/IGF signaling network27. Our data suggest that DAF-16/FOXO is already slightly activated by being cultured in liquid20, and further activation is strongly undesirable, since DAF-16/FOXO promotes broad-spectrum pathogen resistance23.
Finally, it is crucial to use completely sterile worms in any assay that relies on mortality as a readout. If worms are fertile, being in liquid causes difficulties in egg laying. As a result, some of the embryos hatch within the parent, eventually causing its death. For this reason, we generally use a genetic lesion, such as glp-4(bn2)28, that demonstrates a completely penetrant sterile phenotype for these assays. The use of chemicals that prevent embryonic development but still allow egg laying, such as 5-fluorodeoxyuridine (FUdR), should be avoided as they may also interfere with bacterial growth and development.
In general, we have found it quite helpful to plan several time points for analysis. Although the assay is robust and the timing is generally consistent, stochastic and imperceptible changes can shift the timing of death in the assay within 2 – 4 hours. When troubleshooting is necessary, it is often helpful to consider first the simplest of answers; i.e., prepare fresh media, discard the most recent bacterial cultures, and re-streak bacterial strains from frozen stocks, etc. Only very rarely do these measures not restore the assay to functionality.
Due to the relative simplicity of the method, a wide variety of modifications can easily be performed. Changing the worms from a uniform glp-4(bn2) background for high-throughput RNAi screens, such as those we describe herein, is useful for the identification of host response pathways for a wide range of xenobiotics and toxic chemicals. For example, by adding the same chemical or toxin into each well of RNAi plates (e.g., Cry5B toxin, phenanthroline, etc.), pathways that alter the sensitivity to these toxins can be readily identified. These types of screens can also be used to identify defense networks against any of the panoply of pathogens that infect C. elegans.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the Cancer Prevention and Research Institute of Texas (CPRIT) Award RR150044, Welch Foundation Research Grant C-1930, and by the National Institutes of Health K22 AI110552 awarded to NVK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materials
| COPAS FP BioSorter | Union Biometrica | Large object flow cytometer/worm sorter | |
| Cytation 5 | BioTek | ||
| EL406 Washer Dispenser | BioTek | ||
| Multitron Pro | Infors HT | ||
| 24 Deep-Well RB Block | Thermo Fisher Scientific | CS15124 | |
| 384-Well plate | Greiner Bio-One | MPG-781091 | |
| Nematode Growth Media (NGM) | Amount per liter: 18 grams agar, 3 grams NaCl, 2.5 grams Peptone, 1 mL CaCl2 (1 M), 1 mL MgSO4 (1 M), 25 mL Phospate buffer, and 973 mL of milli-Q water | ||
| Slow Killing (SK) plates | Amount per liter: 18 grams agar, 3 grams NaCl, 3.5 grams Peptone, 1 mL CaCl2 (1 M), 1 mL MgSO4 (1 M), 25 mL Phospate buffer, and 973 mL of milli-Q water | ||
| Slow Killing (SK) media | Amount per liter: 3 grams NaCl, 3.5 grams Peptone, 1 mL CaCl2 (1 M), 1 mL MgSO4 (1 M), 25 mL Phosphate buffer, and 973 mL of milli-Q water | ||
| Lysogeny Broth (LB) | USBiological Life Sciences | L1520 | |
| Brian Heart Infusion broth (BHI) | Research Products International Corp | 50-488-526 | |
| Worm Bleach Solution | Amount per 100 mL: 10 mL of 5 M NaOH solution, 20 mL of 5% Sodium Hypochlorite Solution, and 70 mL of sterile water | ||
| S Basal | Amount per liter: 5.85 grams NaCl, 6 grams KH2PO4, 1 gram K2HPO4, and 1 Liter of milli-Q water | ||
| Agar | USBiological Life Sciences | A0930 | |
| NaCl | USBiological Life Sciences | S5000 | |
| Peptone | USBiological Life Sciences | P3300 | |
| CaCl2 | USBiological Life Sciences | ||
| MgSO4 | Fisher Scientific | M63-500 | |
| Phospate buffer | amount per liter: 132 mL of K2HPO4 (1M) and 868 mL of KH2PO4 (1M) | ||
| KH2PO4 | Acros Organics | 7778-77-0 | |
| K2HPO4 | USBiological Life Sciences | P5100 | |
| 5% Sodium Hypochlorite Solution | BICCA | 7495.5-32 | |
| NaOH solution | Fisher Scientific | SS255-1 | |
| Breathe-easy | Diversified Biotech | BEM-1 | |
| SYTOX Orange Nucleic Acid Stain | Fisher Scientific | S11368 | |
| Bacterial Strains | |||
| P. aeruginosa (PA14) | |||
| E. faecalis(OG1RF) | |||
| E. coli superfood (OP50) | |||
| E. coli RNAi expressing bacteria (HT115) | |||
| Worm Strains | |||
| glp-4(bn2) (Beanan and Strome, 1992, PMID: 1289064) | |||
| PINK-1::GFP reporter (Kang et al., 2018, PMID: 29532717) |
References
- Falagas, M. E., et al. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: Characteristics and outcome in a series of 28 patients. International Journal of Antimicrobial Agents. 32 (5), 450-454 (2008).
- Hsueh, P. R., et al. Pandrug-resistant Acinetobacter baumannii causing nosocomial infections in a university hospital, Taiwan. Emerging Infectious Diseases. 8 (8), 827-832 (2002).
- Wang, C. Y., et al. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: clinical features, risk-factors and outcomes. Clinical Microbiology and Infection. 12 (1), 63-68 (2006).
- Yao, Y., et al. Draft genome sequences of pandrug-resistant Serratia marcescens clinical isolates harboring blaNDM-1. Genome Announcements. 5 (3), (2017).
- Utari, P. D., Quax, W. J. Caenorhabditis elegans reveals novel Pseudomonas aeruginosa virulence mechanism. Trends in Microbiology. 21 (7), 315-316 (2013).
- Conery, A. L., Larkins-Ford, J., Ausubel, F. M., Kirienko, N. V. High-throughput screening for novel anti-infectives using a C. elegans pathogenesis model. Current Protocols in Chemical Biology. 6 (1), 25-37 (2014).
- Kirienko, N. V., et al. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host & Microbe. 13 (4), 406-416 (2013).
- Kirienko, D. R., Revtovich, A. V., Kirienko, N. V. A high-content, phenotypic screen identifies fluorouridine as an inhibitor of pyoverdine biosynthesis and pseudomonas aeruginosa virulence. mSphere. 1 (4), (2016).
- Manning, A. J., et al. A high content microscopy assay to determine drug activity against intracellular Mycobacterium tuberculosis. Methods. 127, 3-11 (2017).
- Marwaha, S., et al. N-acylated derivatives of sulfamethoxazole and sulfafurazole inhibit intracellular growth of Chlamydia trachomatis. Antimicrobial Agents and Chemotherapy. 58 (5), 2968-2971 (2014).
- Kota, K. P., et al. Integrating high-content imaging and chemical genetics to probe host cellular pathways critical for Yersinia pestis infection. PLoS One. 8 (1), e55167 (2013).
- Arif, M., et al. Quantification of cell infection caused by Listeria monocytogenes invasion. Journal of Biotechnology. 154 (1), 76-83 (2011).
- Jayamani, E., et al. Characterization of a Francisella tularensis-Caenorhabditis elegans pathosystem for the evaluation of therapeutic compounds. Antimicrobial Agents and Chemotherapy. 61 (9), (2017).
- Moy, T. I., et al. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chemical Biology. 4 (7), 527-533 (2009).
- Rajamuthiah, R., et al. Whole animal automated platform for drug discovery against multi-drug resistant Staphylococcus aureus. PLoS One. 9 (2), e89189 (2014).
- Breger, J., et al. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathogens. 3 (2), e18 (2007).
- Garvis, S., et al. Caenorhabditis elegans semi-automated liquid screen reveals a specialized role for the chemotaxis gene cheB2 in Pseudomonas aeruginosa virulence. PLoS Pathogens. 5 (8), e1000540 (2009).
- Zhou, Y. M., et al. An efficient and novel screening model for assessing the bioactivity of extracts against multidrug-resistant Pseudomonas aeruginosa using Caenorhabditis elegans. Bioscience, Biotechnology, and Biochemistry. 75 (9), 1746-1751 (2011).
- Leung, C. K., Deonarine, A., Strange, K., Choe, K. P. High-throughput screening and biosensing with fluorescent C. elegans strains. Journal of Visual Experiments. (51), (2011).
- Kirienko, N. V., Ausubel, F. M., Ruvkun, G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America. 112 (6), 1821-1826 (2015).
- Kirienko, N. V., Cezairliyan, B. O., Ausubel, F. M., Powell, J. R. Pseudomonas aeruginosa PA14 pathogenesis in Caenorhabditis elegans. Methods in Molecular Biology. 1149, 653-669 (2014).
- Kang, D., Kirienko, D. R., Webster, P., Fisher, A. L., Kirienko, N. V. Pyoverdine, a siderophore from Pseudomonas aeruginosa, translocates into C. elegans, removes iron, and activates a distinct host response. Virulence. , 1-41 (2018).
- Garsin, D. A., et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 300 (5627), 1921 (2003).
- Estes, K. A., Dunbar, T. L., Powell, J. R., Ausubel, F. M., Troemel, E. R. bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 107 (5), 2153-2158 (2010).
- Tjahjono, E., Kirienko, N. V. A conserved mitochondrial surveillance pathway is required for defense against Pseudomonas aeruginosa. PLoS Genetics. 13 (6), e1006876 (2017).
- Moy, T. I., et al. Identification of novel antimicrobials using a live-animal infection model. Proceedings of the National Academy of Sciences of the United States of America. 103 (27), 10414-10419 (2006).
- Henderson, S. T., Bonafe, M., Johnson, T. E. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 61 (5), 444-460 (2006).
- Beanan, M. J., Strome, S. Characterization of a germ-line proliferation mutation in C. elegans. Development. 116 (3), 755-766 (1992).

