Generation of Electronic Cigarette Aerosol by a Third-Generation Machine-Vaping Device: Application to Toxicological Studies
Summary
Electronic cigarette (e-cig) users are increasing worldwide. Little, however, is known about the health effects induced by inhaled e-cig aerosols. This article describes an e-cig aerosol generation technique suitable for animal exposures and subsequent toxicological studies. Such protocols are required to establish experimentally reproducible and standardized e-cig exposure systems.
Abstract
Electronic-cigarette (e-cig) devices use heat to produce an inhalable aerosol from a liquid (e-liquid) composed mainly of humectants, nicotine, and flavoring chemicals. The aerosol produced includes fine and ultrafine particles, and potentially nicotine and aldehydes, which can be harmful to human health. E-cig users inhale these aerosols and, with the third-generation of e-cig devices, control design features (resistance and voltage) in addition to the choice of e-liquids, and the puffing profile. These are key factors that can significantly impact the toxicity of the inhaled aerosols. E-cig research, however, is challenging and complex mostly due to the absence of standardized assessments and to the numerous varieties of e-cig models and brands, as well as e-liquid flavors and solvents that are available on the market. These considerations highlight the urgent need to harmonize e-cig research protocols, starting with e-cig aerosol generation and characterization techniques. The current study focuses on this challenge by describing a detailed step-by-step e-cig aerosol generation technique with specific experimental parameters that are thought to be realistic and representative of real-life exposure scenarios. The methodology is divided into four sections: preparation, exposure, post-exposure analysis, plus cleaning and maintenance of the device. Representative results from using two types of e-liquid and various voltages are presented in terms of mass concentration, particle size distribution, chemical composition and cotinine levels in mice. These data demonstrate the versatility of the e-cig exposure system used, aside from its value for toxicological studies, as it allows for a broad range of computer-controlled exposure scenarios, including automated representative vaping topography profiles.
Introduction
Safety related to the use of electronic cigarettes (e-cigs) is a matter of active debate in the scientific community. On one hand, manufacturers and merchants advertise the potential benefits of e-cigs as a harm reduction product for current smokers, due to the elimination of many harmful substances present in conventional cigarettes, while public health policy decision makers are apprehensive about the absence of data on long-term human health exposures1,2. E-cigs serve at least two distinct purposes, 1) as a replacement vehicle for delivery of nicotine and 2) as a smoking cessation device3. According to the Centers for Disease Control and Prevention (CDC), in 2014, more than 9 million adult Americans used e-cigs on a regular basis. From 2013 to 2014, e-cig use among high school students increased by more than 300%4. Given the rising use of e-cigs among youth as well as in adults1,2,4, and considering the popular, yet unproven, claims about e-cigs as a safer smoking alternative, key scientific questions need to be addressed to determine whether e-cig use poses potential risks to human health, particularly that of the respiratory system1,2. Although e-cigs were first commercialized in the US in 2007, only very limited studies have been carried out on the effects of e-cig aerosol exposures in vitro and on lung structure, function and overall health5,6,7,8,9,10,11. Therefore, in vitro, in vivo and epidemiological data are essential to help establish public policies and regulations related to the consumption of e-cigs. However, production of reliable and reproducible scientific evidence in this emerging field first requires the establishment of standardized e-cig puffing regimes and the generation of reproducible exposure environments in laboratory settings that are reflective of human consumption.
Third-generation e-cig devices, available on the market, are composed of at least one heating coil (atomizer) plus a lithium battery. The e-cig device's power controller can operate at various voltages. These e-cig devices also have a reservoir, into which the e-cig liquid (e-liquid) is introduced. The e-liquid, also known as e-juice, is composed mainly of nicotine, flavors, and carrier solvents (humectants), often propylene glycol (PG), vegetable glycerin (VG) and water. Since, according to the U.S. Food and Drug Administration (FDA), e-liquids are composed of a mixture of "generally regarded as safe" (GRAS) food additive flavoring chemicals and humectants, plus nicotine, they can be considered as safe in food. However, when these liquid formulations are vaped through the e-cig device, they are heated by the atomizer, which changes the physical-chemical properties of the e-liquid, and produces an aerosol or vapor containing carbonyls, more specifically aldehyde compounds12,13. These aldehydes are formed by the thermal degradation and oxidation of glycols, which also yield the formation of hydroxyl radicals14,15,16,17. Those aldehydes which are present in the e-cig aerosol when vaped under specific conditions13, include formaldehyde, acetaldehyde, acetol, acrolein, glycidol, and diacetyl, all of which are known to have potent negative effects on human health, with formaldehyde being a proven human carcinogen15,16,17. In addition, e-cig aerosol also is composed of fine (250 – 950 nm)18,19 and ultrafine (44 – 97 nm)20 particles, which are known to cause pulmonary toxicity through inflammation and oxidative stress mechanisms17. Based on the composition of the e-liquid, i.e., the percentage of individual components present in the formulation, as well as the voltage applied to the e-cig device, which influences the temperature used to vape the e-liquid, the total particulate matter (TPM) concentration of the aerosol will vary, and result in different levels of particles, as well as concentrations of aldehydes, which have been shown to be produced under specific vaping conditions19,21. These aerosols are inhaled by the e-cig users, who control the voltage of their e-cig device. Selection of the voltage is based on personal preferences of nicotine delivery rate, aerosol production, and burning sensation12. Thus, it is imperative to better understand the characteristics of these aerosols in order to provide scientific evidence for adequate regulations governing e-cig and e-liquid manufacturing and consumption policies.
In the context of scientific research, there are several issues that need to be addressed related to 1) the various e-cig device configurations and operation options from which e-cig users can choose; 2) the lack of standardized representative human vaping topography profiles to be used in experimental settings22. This highlights the urgent need to harmonize e-cig research protocols, starting with e-cig aerosol generation and characterization techniques22. The current study focuses on this challenge by describing a detailed step-by-step e-cig aerosol generation technique, with specific experimental parameters considered to be realistic and representative of real-life exposure scenarios. This study also aims to evaluate the influence of voltage on the e-cig aerosol's TPM concentration, as generated using a third-generation vaping device integrated into a commercial computer-controlled exposure system configured for mice whole-body inhalation studies. The description of this experimental protocol, including the generation and characterization of e-cig aerosols, can contribute to the establishment of representative standardized e-cig puffing regimes in a laboratory setting for subsequent toxicological studies.
Protocol
Mice were housed and handled in accord with the NIH Guide for the Care and Use of Laboratory Animals. All procedures and protocols involving mice were approved by the Louisiana State University Institutional Animal Care and Use Committee. The description provided below is specific to the equipment used, as specified in the Table of Materials/Equipment. All air supply was HEPA-filtered.
1. Preparation
- Study & Equipment
- Obtain the necessary approvals (e.g., IACUC) and trainings for the study.
- Set-up the equipment in an adequately ventilated area and become familiar with its operation.
- Gravimetric measurements
- Weigh a clean new 25 mm filter. Record the weight. Place the filter in a cassette.
- Place the cassette, with the filter, in line with a personal sampling pump and a flowmeter adequate to test for a flow of 1 L/min (LPM).
- Electronic cigarette device
- Screw the atomizer into the tank base (Figure 1).
Note: Atomizers containing coils with resistances at 0.15, 0.5 or 1.5 Ω are available. - Critical step: Add a few drops (2 to 3) of e-cig liquid into the atomizer to ensure that the cotton is saturated and will not create a dry burn (Figure 2).
- Insert the tank sleeve into the tank. Then, screw the tank base with the atomizer into the tank sleeve (Figure 1).
- Screw the assembled tank onto the e-cig unit. Make sure the tank opening is facing upwards and put the cover in place on top of the tank (Figure 1).
- Put the e-cig unit on its base-plate by rotating the plunging arm of the solenoid valve. When in place, rotate it back into place so that it can align with the trigger button on the e-cig unit.
- Connect the end of the e-cig unit to the lower part of the condenser via a two-way valve attachment and a piece of tubing (Figure 3).
- Ensure that the upper end of the condenser is connected correctly to the aerosol generating system and aerosol exposure chamber via proper tubing.
- Critical step: Verify that the aerosol concentration measurement instrument is in place at the exit of the aerosol exposure chamber.
- Critical step: Remove the tank cover and fill the tank with 10 mL of e-cig liquid. Replace the tank cover.
Note: This volume is sufficient for a 2-h exposure period.
- Screw the atomizer into the tank base (Figure 1).
2. Exposure
- Software connection
- On the day of the experiment, turn on the computer. Remember to also turn ON the aerosol concentration measurement instrument by manually pressing the power button.
- Launch the operating software. Click on Experimentation Session. Select the appropriate study. Choose the Template for the e-cig experiment.
- In the New Experiment Window, enter a name for the experimental session. In the Experiment Properties Window, type in the operator initials in the operator box. Click OK.
- Channel calibration
- Follow the steps in the calibration wizard in order to adequately calibrate the aerosol generation system.
- Step 1: Click Next on the Channel Calibration window after confirming that there is a check mark in the aerosol concentration measurement instrument (MicroDust Pro) box.
- Step 2: In Apply Value Window, click Next. Step 3: Enter the Target Value Input as 0 g/m3. Step 4: Place the T-shaped calibration insert into the slot to complete the calibration process and press Next to get to the following window.
- Enter the value read on the aerosol concentration measurement instrument. Press Next after entering this value. Review the calibration Results Window and click Next.
- Final Step: In Calibration Complete Window, click Finish. For System Flow Test, in test window, test pumps 1 and 2 (refer to the user manual).
- Confirm – “Would you like to start recording continuous data?”, click Yes. Confirm – “Would you like to start the default profile?”, click Yes.
- Follow the steps in the calibration wizard in order to adequately calibrate the aerosol generation system.
- Electronic cigarette aerosol exposure
- If doing an in vivo inhalation study, place the mice in the whole-body exposure chamber(s) at this time.
- Immediately go to the Profiles Window and right-click on the desired profile, scroll down to Start task to initiate a bias flow of fresh air inside the exposure chamber(s).
- When ready to initiate the e-cig aerosol generation and exposure experiment, right-click on the desired profile in the Profiles Window, scroll down to Start task and left click to select (Figure 4).
- Critical step: Record the concentration measured by the aerosol concentration measurement instrument. The concentration should be > 0 mg/m3.
Note: The device operating principle is based on optical detection and is used in this system to provide a qualitative assessment in real time of the exposure levels in the chamber. - Ensure that e-liquid is available in tank during the entire duration of the exposure.
- To stop the experiment after reaching the desired exposure duration, right click on the profile, scroll down to Stop profile, and left click to select. Ensure that the bias flow is initiated immediately following the completion of the exposure profile.
- Remove the subjects (animals) from the exposure chamber and return them to their housing cage and room.
3. Post-Exposure Analysis
- At the end of the experimental session, close the operating software and turn OFF the aerosol concentration measurement device.
- Detach the cassette with the filter from the pump and record the time when it was removed. Place the filter in a desiccator and allow the filter to dry for at least 48 h (preferably 96 h). Then, weigh the filter with the accumulated e-cig aerosol particles and record the weight.
- Calculate the total particulate matter (TPM) concentration in terms of mass per puff23.
- Record the mass accumulated on the filter. Calculate the total volume sampled during the exposure period using the sampling duration and the pump flow.
- Divide the mass collected on the filter by the volume of air.
Note: TPM concentration is expressed in weight per volume units. Divide the TPM concentration by the total number of puffs generated by the e-cig profile used.
4. Cleaning and Maintenance
- Pour out the e-liquid from the e-cig tank and empty the condenser using the attached syringe. Ensure that the atomizer coil did not burn during the experiment. Change the atomizer coil after each experiment.
- Clean the pumps after each experiment. Detach the pump heads and remove the connectors and valves. Wipe off any excess e-liquid or accumulated moisture using either a cotton swab or tissue.
- Clean the whole-body exposure chambers. Follow the manufacturer’s instructions and remove any condensed e-liquid from all surfaces.
Note: It is recommended to avoid the use of alcohol as it may cause irreversible damage.
Representative Results
Table 1 shows the characteristics of the exposure environment inside a 5-L whole-body chamber following e-cig aerosol generation. These data are the results of a 2-h exposure session with only the carrier solvents e-liquid base, i.e., 50/50 ratio of PG and VG in the absence of flavoring or nicotine. The aerosol was produced by a third-generation battery-powered e-cig device with a 0.5 Ω resistance. A total of seven e-cig voltages were tested with a topography profile of 70-mL puff volume, 3-s puff duration, and 1-min intervals. As expected, increasing e-cig voltage leads to higher TPM concentrations of aerosol in the exposure chamber used, as reported with the gravimetrically calculated mass (mg) per puff. However, the changes in TPM concentration follow a somewhat sigmoidal pattern over the voltage range studied. The relationship between the voltage and the TPM concentration is initially linear from 1.8 to 3.2 V, and displays an exponential jump with a subsequent plateau between 3.2 to 4.8 V.
Figure 5 shows the results of a physical characterization of the e-cig aerosols inside the whole-body exposure chamber. Particle number concentration and size distribution were measured under varied experimental conditions using a scanning motility particle sizer. A wide range of mass and number concentrations, as well as particle size distributions, mostly composed of fine and ultrafine particles, can be achieved by using various predefined or user-defined automated puffing profiles that can be adjusted or modified via the software (Figure 6), as well as e-cig device design options (i.e., atomizer coil resistance or battery voltage). These results highlight the versatility of the exposure system used to simulate, in an experimental setting, a wide range of possible human e-cig topography profiles.
As an example, an experimental e-cig exposure environment was created based on current information regarding e-cig consumers' personal preferences and was subsequently characterized (Table 2). Here, the e-cig device was equipped with a coil atomizer of 0.5 Ω and operated at 3.2 V. The topography profile used consisted of a 55-mL puff volume, 3-s puff duration and 30-s intervals while the e-liquid tested included the carrier solvents (i.e., PG and VG at a 50/50 ratio), alone and in combination with 36 mg/mL nicotine and cinnamon flavor (Table 2). Over a 2-h exposure period, this exposure profile draws a larger number of puffs and allows for a higher total volume to be sampled in comparison to the previously employed 70-mL, 1 puff per min profile (13,200 mL versus 8,400 mL, respectively). Consequently, a smaller average particulate mass per puff is obtained under this topography profile for a same voltage and similar power (Tables 1, 2). The results seem to indicate that the presence of nicotine and cinnamon flavor in the e-liquid may have a negative effect on the particulate mass per puff. However, the difference between the two experimental conditions did not reach the level of statistical significance.
The results of a chemical analysis of the e-cig aerosol generated with the latter topography profile (55-mL puff volume, 3-s puff duration and 30-s intervals) are shown in Table 3 and Figure 7. A total of 82 puffs of e-cig aerosol generated under 3.2 V with an e-liquid composed of 50/50 ratio of PG and VG, 36 mg/mL nicotine, and cinnamon flavor were sampled onto silica-based filters that were subsequently used for the chemical characterization of the e-cig emission by GC/MS techniques. This sample was collected right after the condenser. The analysis revealed that, in addition to nicotine and cinnamaldehyde that were expected, other compounds such as acrolein, catechol, and benzothiazole were identified in the e-cig aerosol. These chemicals are known respiratory irritants and show the complexity of the aerosol composition once the e-liquid is heated and aerosolized.
In addition to e-cig aerosol physico-chemical characterization, the e-cig generator and exposure system employed is also suitable for animal exposures. As illustrated in Figure 8, the concentration of serum cotinine, a major metabolite of nicotine, can be used to monitor or confirm exposures to e-cig aerosol from nicotine-containing e-liquids in mice. In the present example, the mice exposed to e-cig aerosol displayed a significant increase in their serum cotinine concentration.
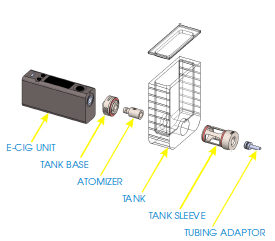
Figure 1. E-cig generator deconstructed view. Image shows the various elements composing the e-cig generator (e-cig unit, tank base, atomizer, tank, tank sleeve, tubing adaptor).
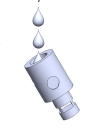
Figure 2. E-cig generator atomizer. Image of where to put e-cig liquid into atomizer.
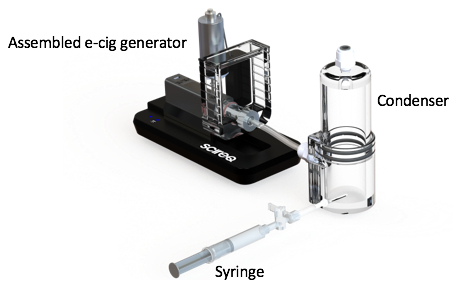
Figure 3. E-cig overall view. Image shows assembled e-cig generator with extension, including the condenser.
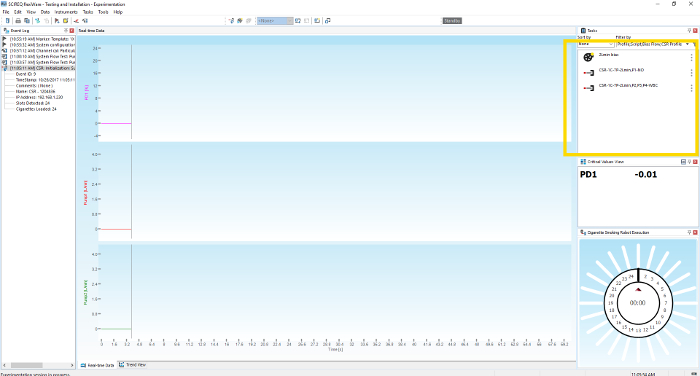
Figure 4. E-cig generator operating software. Image shows the selection of vaping profile on the software. Please click here to view a larger version of this figure.
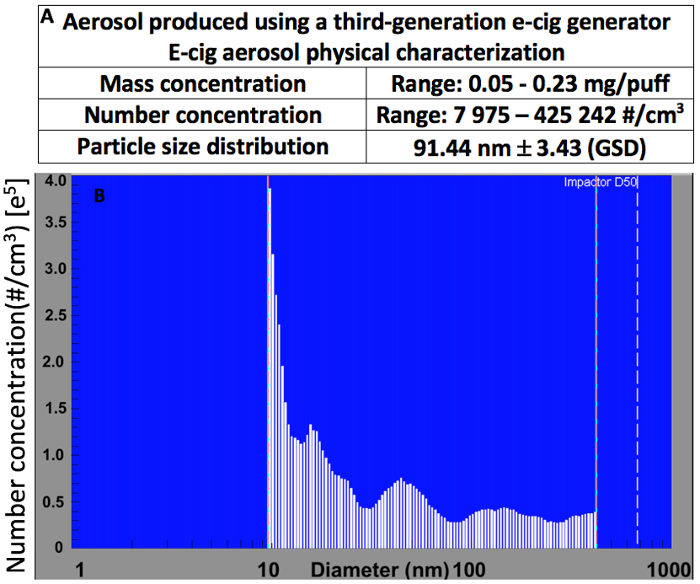
Figure 5. Representative physical characterization of e-cig aerosols produced by a third-generation e-cig generator in a 5 L chamber shows (A) the impact of the e-cig device power (6-40 W) on the exposure conditions that can be generated and (B) that e-cig aerosols are composed of fine & ultrafine particles. Particle number concentration and size distribution measured using a scanning mobility particle sizer. Exposure parameters: atomizer's resistance 0.5 Ω and voltage varying from 1.8 to 4.8 V; vaping under a topography profile of either 3 s puff duration, 70-mL puff volume every 60 s or 3 s puff duration, 55 mL puff volume every 30 s; using an e-liquid composed of PG and VG at a 50/50 ratio. Please click here to view a larger version of this figure.
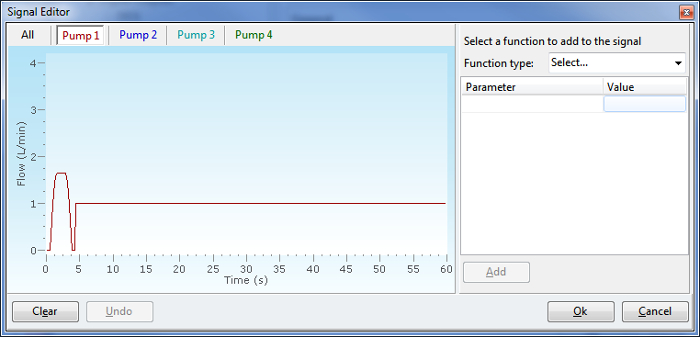
Figure 6. Automated puffing profiles can be created, adjusted or modified via the software. Images shows one step of the Profile Creation Wizard that is used to enter key vaping topography factors, including puff volume, puff duration, puff interval, and puff profile. Please click here to view a larger version of this figure.
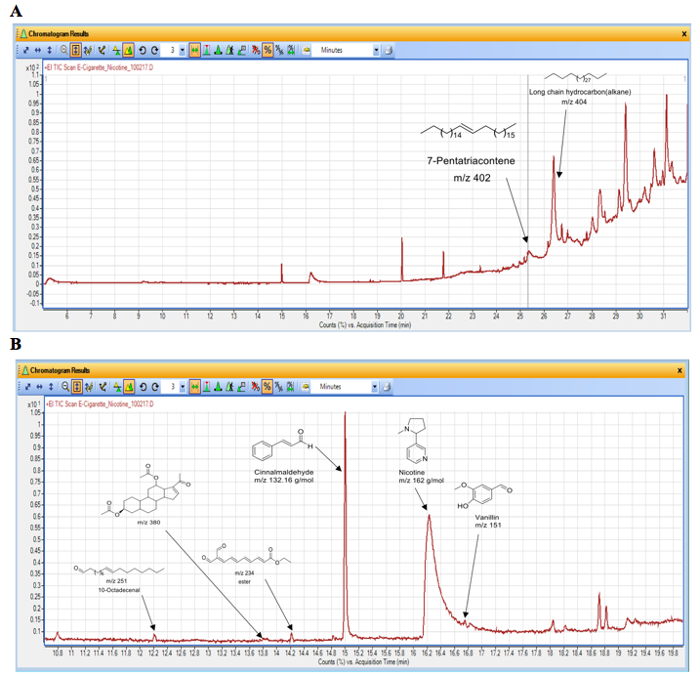
Figure 7. Spectrum of GC/MS results for the e-cig aerosol. As described in Table 3, e-cig aerosol was produced using the e-cig device with a 0.5 Ω coil atomizer set at 3.2 V vaping under a topography profile of 55 mL puff volume, 3 s puff duration and 30 s intervals with an e-liquid composed of 50/50 ratio of PG and VG, 36 mg/mL nicotine and cinnamon flavor. A sample of 82 puffs of e-cig aerosol was collected right after the condenser onto a silica-based filter, which was subsequently used for chemical analysis by gas chromatography – mass spectrometry (GC/MS) techniques. (A) Whole spectrum; (B) Zoom in. Please click here to view a larger version of this figure.
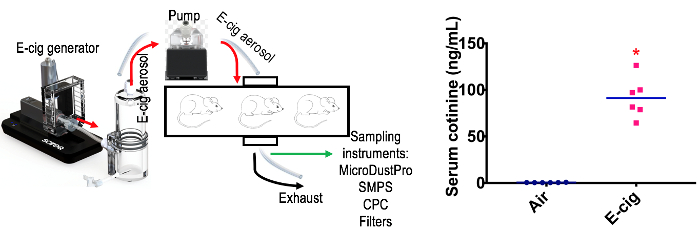
Figure 8. Schematic of e-cig exposure system for animal studies. The whole-body e-cig aerosol inhalation system (A) is suitable for animal exposures, with cotinine levels in e-cig exposed male BALB/C mice (B) that are comparable to the levels of mainstream cigarette smoke exposures. Air group cotinine levels 0.3-1.2 ng/mL. N = 6 per group, *p < 0.05. Mouse/rat cotinine ELISA. Exposure parameters: atomizer's resistance and battery voltage set at 1.5 Ω and 4.2 V, respectively; vaping under a topography profile of 3 s puff duration, and a 55 mL puff volume every 30 s; using an e-liquid composed of 36 mg/mL of nicotine, cinnamon flavor and a 50/50 PG/VG ratio. Mice were exposed to a TPM concentration of 0.12 ± 0.09 mg/puff of e-cig aerosol for 2 h/day for 28 days, while controls were exposed to filtered-air. Please click here to view a larger version of this figure.
| E-cig Voltage (V) | E-cig Power (W) | Mass per puff (mg) | Chamber | Chamber Temperature (oC) |
| Relative Humidity (%) | ||||
| 1.8 | 6.3 ± 0.3 | 0.005 ± 0.004 | 46.0 ± 3.3 | 23.7 ± 0.6 |
| 2.3 | 8.8 ± 0.1 | 0.009 ± 0.005 | 27.8 ± 9.1 | 24.0 ± 0.6 |
| 2.55 | 10.6 ± 0.2 | 0.021 ± 0.008 | 53.2 ± 1.2 | 23.2 ± 0.2 |
| 2.8 | 12.4 ± 0.3 | 0.061 ± 0.073 | 51.3 ± 1.1 | 24.2 ± 0.6 |
| 3.2 | 15.8 ± 0.6 | 0.065 ± 0.013 | 56.6 ± 2.3 | 23.1 ± 0.2 |
| 3.7 | 23.3 ± 0.6 | 0.741 ± 0.417 | 51.2 ± 5.5 | 23.6 ± 0.5 |
| 4.8 | 40.4 ± 1.3 | 0.823 ± 0.198 | 25.4 ± 7.7 | 23.7 ± 0.5 |
Table 1. E-cig device parameters tested and exposure conditions in a 5 L exposure chamber with a 0.5 Ω coil atomizer. Topography profile for a 2 h exposure: 70 mL puff volume, 3 s puff duration and 1 min intervals, using only carrier solvents e-liquid base, i.e., 50/50 ratio PG and VG. All voltages were tested in triplicate (n = 3). Data are expressed as mean ± standard deviation (SD).
| E-cig Voltage (V) | E-cig Power (W) | E-Liquid nicotine (mg/mL) | E-Liquid flavor | Mass per puff (mg) | Chamber | Chamber Temperature (oC) |
| Relative Humidity (%) | ||||||
| 3.2 | 16.6 ± 0.2 | 0 | None | 0.273 ± 0.184 | 47.4 ± 3.9 | 23.6 ± 0.2 |
| 3.2 | 15.9 ± 1.3 | 36 | Cinnamon | 0.102 ± 0.078 | 59.6 ± 3.1 | 22.7 ± 0.2 |
Table 2. E-cig device parameters tested and exposure conditions in a 5 L exposure chamber with a 0.5 Ω coil atomizer. Topography profile for a 2 h exposure: 55 mL puff volume, 3 s puff duration and 30 s intervals, using 1) only carrier solvents e-liquid base, i.e., 50/50 ratio PG and VG, and 2) e-liquid base + nicotine (36 mg/mL) and cinnamon flavoring. The two e-liquids were tested in triplicate (n = 3). Data are expressed as mean ± SD.
| List of compounds in e-cig aerosol |
| 2-Propenal (acrolein) |
| 7-Pentatriacontene |
| 10-Octadecenal |
| Benzothiazole |
| Catechol |
| Cinnamaldehyde |
| Ethoxy acetic acid |
| Nicotine |
| Vanillin |
Table 3. Non-exhaustive list of compounds found in the e-cig aerosol. E-cig aerosol was produced using the e-cig device with a 0.5 Ω coil atomizer set at 3.2 V vaping under a topography profile of 55 mL puff volume, 3 s puff duration and 30 s intervals with an e-liquid composed of 50/50 ratio of PG and VG, 36 mg/mL nicotine and cinnamon flavor. A sample of 82 puffs of e-cig aerosol was collected right after the condenser onto a silica-based filter, which was subsequently used for chemical analysis by gas chromatography – mass spectrometry (GC/MS) techniques.
Discussion
A major unanswered question is whether long-term exposure to e-cig aerosol results in pulmonary toxicity. In addition, the general safety of e-cigs regarding human health is still a matter of controversy. In August 2016, the U.S. FDA expanded its regulatory authority on all tobacco products, including e-cigs. E-cig research, however, is challenging and complex due mostly to 1) the absence of standardized assessments; 2) the wide variety of e-cig devices (~2,800 different models from 466 identified brands)24; 3) over 7,700 unique e-liquid flavors24; 4) the various possible combinations of humectant ratios. Given the complexity of the field, it is essential, in order to face the challenge and generate sound scientific evidence, that careful considerations to the experimental conditions and reproducible processes are employed. In the present study, the focus was put on the description of an e-cig aerosol generation technique that can enable investigators to obtain unique data sets related to realistic and comprehensive e-cig aerosol exposure-related effect continuums. These can be of timely relevance to address e-cig-related safety or toxicity questions for the establishment of regulations on e-cig design features that potentially can have a direct impact on public health policies.
In the present article, meaningful exposure environments were generated using a computer-controlled system able to integrate the latest generation of e-cig devices as well as allowing for predefined or user-defined automated puffing profiles and set operating conditions (e.g., constant power source, standard values of resistance, voltage, or temperature). These automated puffing profiles include the standard conditions: 55 mL puff volume, 3 s puff duration, 30 s puff interval, and square puff profile, from the "Routine analytical machine for e-cigarette aerosol generation and collection – Definitions and standard conditions" provided by the Coresta Recommended Method (CRM) N°8125 (Table 2). Since the system used can generate various automated puffing profiles, it also complies with ISO 20768 (Vapour products – Routine analytical vaping machine – Definitions and standard conditions)26 puffing regime requirements. As expected, e-cig puffing regime standard conditions contrast with the ones from ISO 330827, which defines the standard conditions for cigarette-smoking machines (35 mL puff volume, 2 s puff duration, 60 s puff interval, and bell puff profile). These differences between cigarette smoking patterns and e-cig vaping patterns among users are well established28. In the present study, the examples and data provided show that aerosols generated from this system and a third-generation e-cig device with adjustable voltage produce high TPM concentrations, reaching up to 0.27 and 0.82 mg per 55 and 70 mL puff, respectively. E-cig aerosols at these concentrations were collected right after the exposure chamber (Table 1-2, Figure 5). The results also show that there is more than a 160-fold difference in the particulate mass per puff produced with voltages varying from 1.8 to 4.8 V (Table 1). This voltage range is characteristic of the operating settings of e-cig devices on the U.S. market, which allow for the application of voltage ranging from 2.9 to 5.2 V29. The results are also consistent with previously published data18,21 where high levels of TPM collected at the outlet of the e-cig generator were reported for similar topography profiles (1.4 to 5.8 mg/puff). Critical steps within the protocol include adding a few drops of e-liquid to the atomizer prior to each exposure session to ensure a) that no dry burn is produced; b) e-liquid is available in the tank during the entire duration of the exposure; and verify that the e-cig aerosol is generated as expected by taking regular readings on the real-time concentration measurement device. It is well established that e-cig users try to avoid dry puffs, which occur in dry burn conditions. This vaping condition is related to the formation of high levels of aldehydes, including formaldehyde, a known carcinogen and respiratory toxicant13,30. Therefore, ensuring that this condition is avoided during the exposures is crucial. Finally, in terms of nicotine exposure, mice exposed to e-cig aerosol from a 36 mg/mL nicotine-containing e-liquid for 2 h per day for 28 days (levels of 0.12 mg/puff) presented serum cotinine concentrations of 91 ng/mL (Figure 8); a level similar to that of cigarette smokers (> 100 ng/mL)31,32,33, which is even lower than that of regular e-cig users (median saliva cotinine of 252 ng/mL)34. It was reported in a vaping topography study that 235 was the maximum number of puffs per day taken by e-cig users35,36. This is very similar to our exposure profile producing 1 puff every 30-sec for 2-h per day (total of 240 puffs). Thus, this vaping topography profile models e-cig users daily puff consumption and behavior.
Over the past decade, e-cig devices evolved from first-generation, cigarette-like, single-use, low-powered devices, to second-generation removable and refillable tank style devices, and now to third-generation tank-style devices with customizable features24 for 1) the atomizer's coil resistance: the element responsible for heating the e-liquid, and 2) the power controller, which a) can operate at various voltages, b) affects the temperature of the heating element and c) determines whether or not the boiling temperature of the solution is reached24,37. During e-cig use, the e-liquid is typically heated at 200 °C or greater38, and it is in the aerosol form that its constituents interact with biological matrices. Therefore, the characterization of e-cig aerosol is essential. E-liquids solvents differ in volatility such that solutions composed mainly of PG (70%), which are less viscous and evaporate at a lower temperature37, produce aerosols with relatively smaller particles that increase the user's 'throat hit' experience20. On the other hand, VG-based e-liquids aerosolize at higher temperatures37 and produce aerosols with relatively larger particles which, from a user's experience, increases the flavor and the amount of vapor generated5,17,39. Thus, it has previously been established that the PG/VG ratio of the e-liquid influences the size distribution of the particles present in the e-cig aerosol19,20. As shown in Figure 5, using an e-liquid composed of a 50/50 PG/VG ratio, e-cig aerosols with median diameters of ~ 100 nm were obtained. These results are in the same range as those reported by Baassiri, et al.20. This suggests that in addition to the e-liquid base, the exposure parameters, including the e-cig operating settings (resistance, voltage, and power) and puffing profile, may affect the physical characteristics of the aerosols produced. Moreover, the nicotine concentration and flavoring chemicals added to the e-liquid base also can potentially influence the e-cig aerosol physicochemical properties. It was previously shown that an e-liquid that is less viscous produces an aerosol composed of finer particles, resulting in a less dense vapor, yielding a lower TPM concentration17. Using the same PG/VG ratio for both e-liquids tested, the e-liquid containing 36 mg/mL of nicotine and cinnamon flavoring chemical, implying that it is more diluted than the e-liquid base only (PG/VG + nicotine + cinnamon flavor versus PG/VG alone), appeared less viscous than the e-liquid composed solely of PG and VG. The apparent difference in viscosity between the two e-liquids may explain the disparity in the mass per puff obtained under equal e-cig vaping settings (Table 2). However, lower TPM may not correlate with less harmful aerosol, since the particle size distribution and the chemical characterization of the aerosol must also be considered. Indeed, the thermal degradation of VG and the chemical interactions of the e-liquid components produce emissions of harmful aldehydes, including formaldehyde and acetaldehyde, known to be potent threats to human health15,17,40. As noted in Table 3, the chemical analysis of the e-cig aerosol produced here revealed that it also contained acrolein, monochlorophenol, catechol and benzothiazole. All are known respiratory irritants, while catechol is additionally classified as possibly carcinogenic to humans (group 2B) according to the International Agency on Research on Cancer (IARC)41,42,43. This adds to the effects related to the chemistry of the flavoring agent incorporated into the e-liquid. For example, cinnamaldehyde and diacetyl, two of the Flavor and Extract Manufacturers Association high-priority flavoring chemicals for respiratory hazard, when inhaled by workers, have been shown to impair lung function and cause irreversible lung damage (bronchiolitis obliterans, namely 'popcorn lung')44. Cinnamaldehyde has been shown to be highly cytotoxic in vitro45,46,47 and is very popular in e-liquids48. In the current study, the presence of cinnamaldehyde was identified in the e-cig aerosol from the cinnamon flavored e-liquid (Table 3 and Figure 7). Overall, this demonstrates the need to analyze e-cig aerosols for both, physical and chemical characteristics.
As mentioned above, the exposure technique described here can be extremely versatile. It can allow for the modifications of the puffing regime (via the software), of the operating features of the e-cig device or even of the type of exposure chamber (nose-only and whole-body) (via the hardware). This provides the investigator with all the flexibility to adapt or adjust the experimental conditions to the need of each research project. Troubleshooting this technique includes ensuring that the connections between the e-cig condenser, tubes, pumps and chambers are adequately secured, and that all chambers are properly sealed (for more detailed information refer to user manual). As noted and as tested in this study, a variety of factors can influence e-cig aerosol production and composition22. These factors are associated with the ratios and constituents of the e-liquid formulation, which impact the chemical component of the aerosol, as well as the selected e-cig device characteristics and operation settings, which influence the heating conditions used to aerosolize the e-liquid, and thus the composition as well as the physical component of the aerosol. E-liquids are composed of GRAS food additives, however, their safety following heating and aerosolization has not been established. Most importantly, e-cig users inhale these aerosols and control the puffing profile as well as the choice of both e-liquid and the operating settings (resistance and voltage) of their e-cig devices. These are key factors which can significantly impact the e-cig aerosol emissions and should therefore be carefully controlled and reported in experimental research.
As most experimental methods, the present e-cig exposure technique has advantages and limitations. While versatile and well suited for toxicological studies, it is also known that mice are nose-breathers and that whole-body exposures may also allow for dermal and oral absorption in addition to the inhalation exposure route. The advantages and disadvantages of using whole-body and nose-only inhalation exposures have been described extensively elsewhere49,50. While nose-only exposures more closely mimic the inspiration/expiration patterns that govern the transport and deposition of particles in the respiratory tract, this mode of exposure is more stressful to the animals and is not adequate for long-term inhalation studies using large number of animals49. In addition, the studies which compared whole-body and nose-only exposures in rodents exposed by inhalation to the same toxicant under the same exposure conditions (TiO2 nanoparticles, cigarette smoke) found no statistical difference between those two modes of exposure for lung particle deposition and lung responses50,51. Since the effects induced by chronic exposures to e-cig aerosol are largely undocumented and under-investigated, the e-cig exposure system described in this manuscript is useful for bridging this knowledge gap. Also, the third-generation machine-vaping device used in this study is oriented in a horizontal configuration. There is a possibility that the orientation of the device could have an effect on aerosol production; however, to the best of our knowledge, for third-generation e-cig devices, the orientation variable has not been tested previously. The horizontal orientation is the preferred position for beginner users of e-cig. This helps promote better wicking and minimizes the risks of e-liquid leaking. Thus, the horizontal orientation is representative of vaping behaviors of populations of e-cig users and has been used by other research groups21. It is also important to note that the power displayed on the e-cig device may slightly differ from the actual power supplied to the device22,52, and that therefore it also may be advisable to measure the power supply values externally or use a corded power supply for a steady supply of energy.
There is a substantial research and knowledge gap for biomarkers of toxicity associated with long-term exposure to e-cig aerosols. This exposure system represents a step forward in this field by allowing the investigators to determine the effects of long-term inhalation exposures of animals to aerosolized e-cig liquid. Other existing e-cig exposure methods also have the capability for investigating the impact of puffing regime and operating settings of e-cig devices on toxicological endpoints19,20,22,53. These exposure systems will help provide scientific evidence for future regulations on new alternative tobacco products. Ultimately, well-conducted and suitable toxicological studies will help better inform the policymakers, healthcare providers and the 9 million Americans that are e-cig users4. Most importantly, exposure systems that do not reproduce real-life vaping scenarios should be avoided. E-liquids are typically heated at 200 °C or greater temperatures38 in an e-cig device, therefore, scenarios where the e-liquid is simply nebulized, or warmed to 37 °C and then nebulized8, should not be considered as representative of e-cig users consumption. Currently, e-cig consumers may reach potentially harmful e-cig aerosol constituent levels by using design features of third-generation e-cig devices that allow for the adjustment of distinctive heating conditions via changes in the atomizer's coil resistance and the battery voltage. Therefore, more experimental studies are needed to determine the health effects related to chronic inhalation exposures to e-cig aerosols. This begins by establishing reproducible and standardized e-cig exposure systems25,26. Thus, having a versatile e-cig exposure system that allows for a broad range of exposure scenarios, including automated representative vaping topography profiles, is an asset to the conduct of experimental studies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This project was supported by a grant (AP) from the Louisiana Governor's Biotechnology Initiative GBI-BOR#013, as well as by Louisiana State University, School of Veterinary Medicine faculty start-up funds (AN).
Materials
| inExpose complete solution – for electronic cigarette aerosol delivery to a 5L whole-body chamber, including eVic-VTC Mini (e-cig device, Joyetech) | SCIREQ Scientific Respiratory Equipment Inc. | ||
| flexiWare software | SCIREQ Scientific Respiratory Equipment Inc. | FW8 | |
| Computer | Dell | Core 2 Duo | |
| Tygon | Tygon | R-3603 | |
| MicroDust Pro | Cassella | 176000A | |
| Personal sampling pump | Sensidyne | Gilian BDX II | |
| Glass fiber filter | Millipore | AP4002500 | |
| Sampling cassette | Made in house | ||
| Flow meter | TSI Inc. | 4100 series | |
| Electronic cigarette liquid (e-juice) | Local vape shop | ||
| Scanning mobility particle sizer | TSI Inc. | 3080 | |
| Microbalance | Sartorius | MC5 Micro Balance |
References
- Baeza-Loya, S., et al. Perceptions about e-cigarette safety may lead to e-smoking during pregnancy. Bulletin of the Menninger Clinic. 78 (3), 243-252 (2014).
- Kahr, M. K., et al. A qualitative assessment of the perceived risks of electronic cigarette and hookah use in pregnancy. BMC Public Health. 15, 1273 (2015).
- Mark, K. S., Farquhar, B., Chisolm, M. S., Coleman-Cowger, V. H., Terplan, M. Knowledge, Attitudes, and Practice of Electronic Cigarette Use Among Pregnant Women. Journal of Addiction Medicine. 9 (4), 266-272 (2015).
- Larcombe, A. N., Janka, M. A., Mullins, B. J., Berry, L. J., Bredin, A., Franklin, P. J. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. American Journal of Physiology Lung Cell Molecular Physiology. 313 (1), L67-L79 (2017).
- Neilson, L., Mankus, C., Thorne, D., Jackson, G., DeBay, J., Meredith, C. Development of an in vitro cytotoxicity model for aerosol exposure using 3D reconstructed human airway tissue; application for assessment of e-cigarette aerosol. Toxicology In Vitro. 29 (7), 1952-1962 (2015).
- Leigh, N. J., Lawton, R. I., Hershberger, P. A., Goniewicz, M. L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS). Tobacco Control. 25 (Suppl 2), ii81-ii87 (2016).
- Garcia-Arcos, I., et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax. 71 (12), 1119-1129 (2016).
- Vardavas, C. I., Anagnostopoulos, N., Kougias, M., Evangelopoulou, V., Connolly, G. N., Behrakis, P. K. Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest. 141 (6), 1400-1406 (2012).
- Pichelstorfer, L., Hofmann, W., Winkler-Heil, R., Yurteri, C. U., McAughey, J. Simulation of aerosol dynamics and deposition of combustible and electronic cigarette aerosols in the human respiratory tract. Journal of Aerosol Science. 99, 125-132 (2016).
- Sosnowski, T. R., Kramek-Romanowska, K. Predicted deposition of e-cigarette aerosol in the human lungs. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 29 (3), 299-309 (2016).
- Kosmider, L., et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine & Tobacco Research. 16 (10), 1319-1326 (2014).
- Farsalinos, K. E., Voudris, V., Poulas, K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction. 110 (8), 1352-1356 (2015).
- Geiss, O., Bianchi, I., Barahona, F., Barrero-Moreno, J. Characterization of mainstream and passive vapours emmited by selected electronic cigarettes. International Journal of Hygiene and Environmental Health. 218 (1), 169-180 (2015).
- Geiss, O., Bianchi, I., Barrero-Moreno, J. Correlation of volatile carbonyl yields emitted by e-cigarettes with the temperature of the heating coil and the perceived sensorial quality of the generated vapours. International Journal of Hygiene and Environmental Health. 219 (3), 268-277 (2016).
- Flora, J. W., et al. Method for the Determination of Carbonyl Compounds in E-Cigarette Aerosols. Journal of Chromatographic Science. 55 (2), 142-148 (2017).
- Sleiman, M., et al. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environmental Science & Technology. 50 (17), 9644-9651 (2016).
- Ingebrethsen, B. J., Cole, S. K., Alderman, S. L. Electronic cigarette aerosol particle size distribution measurements. Inhalation Toxicology. 24 (14), 976-984 (2012).
- Pouchez, J., et al. Impact of power level and refill liquid composition on the aerosol output and particle size distribution generated by a new-generation e-cigarette device. Aerosol Science & Technology. 52 (4), 359-369 (2018).
- Baassiri, M., et al. Clouds and "throat hit": effects of liquid composition on nicotine emissions and physical characteristics of electronic cigarette aerosols. Aerosol Science & Technology. 51 (11), 1231-1239 (2017).
- Gillman, I. G., Kistler, K. A., Stewart, E. W., Paolantonio, A. R. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regulatory Toxicology and Pharmacology. 75, 58-65 (2016).
- Soulet, S., Pairaud, C., Lalo, H. A novel vaping machine dedicated to fully controlling the generation of e-cigarette emissions. International Journal of Environmental Research and Public Health. 14 (10), 1225 (2017).
- Zhu, S. H., et al. Four hundred and sixty brands of e-cigarettes and counting: implications for product regulation. Tobacco Control. 23 (Suppl 3), iii3-iii9 (2014).
- . CORESTA Recommended Method No81. Routine analytical machine for e-cigarette aerosol generation and collection – definitions and standard conditions Available from: https://www.coresta.org/sites/default/files/technical_documents/main/CRM_81.pdf (2015)
- . ISO/FDIS 20768. Vapour products – Routine analytical vaping machine – Definitions and standard conditions Available from: https://www.iso.org/standard/69019.html (2018)
- . ISO 3308:2000(E). Routine analytical cigarette-smoking machine – Definitions and standard conditions Available from: https://www.iso.org/standard/28325.html (2018)
- St-Helen, G., Ross, K. C., Dempsey, D. A., Havel, C. M., Jacob, P., Benowitz, N. L. Nicotine delivery and vaping behavior during ad libitum e-cigarette access. Tobacco Regulatory Science. 2 (4), 363-376 (2016).
- Talih, S., et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine & Tobacco Research. 17 (2), 150-157 (2015).
- Korzun, T., et al. E-cigarette airflow rate modulates toxicant profiles and can lead to concerning levels of solvent consumption. ACS Omega. 3 (1), 30-36 (2018).
- Benowitz, N. L., Bernert, J. T., Caraballo, R. S., Holiday, D. b., Wang, J. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the Unites States between 1999 and 2004. American Journal of Epidemiology. 169 (2), 236-248 (2009).
- Sussan, T. E., et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 10 (2), e0116861 (2015).
- Flouris, A. D., et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology. 25 (2), 91-101 (2013).
- Etter, J. F. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug and Alcohol Dependence. 160, 218-221 (2016).
- Dawkins, L., Turner, J., Roberts, A., Soar, K. ‘Vaping’ profiles and preferences: an online survey of electronic cigarette users. Addiction. 108 (6), 1115-1125 (2013).
- Logue, J. M., et al. Emissions from Electronic Cigarettes: Assessing Vapers’ Intake of Toxic Compounds, Secondhand Exposures, and the Associated Health Impacts. Environmental Science & Technology. 51 (16), 9271-9279 (2017).
- Talih, S., et al. Transport phenomena governing nicotine emissions from electronic cigarettes: model formulation and experimental investigation. Aerosol Science & Technology. 51 (1), 1-11 (2017).
- Canistro, D., et al. E-cigarettes induce toxicological effects that can raise the cancer risk. Scientific Report. 7, 2028 (2017).
- Chen, Z., Zeng, D. D. Mining online e-liquid reviews for opinion polarities about e-liquid features. BMC Public Health. 17, 633 (2017).
- Dinakar, C., O’Connor, G. T. The health effects of electronic cigarettes. New England Journal of Medicine. 375 (14), 1372-1381 (2016).
- Schweigert, N., Zehnder, A. J. B., Eggen, R. I. L. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environmental Microbiology. 3 (2), 81-91 (2001).
- Ginsberg, G., Toal, B., Kurland, T. Benzothiazole toxicity assessment in support of synthetic turf field human health risk assessment. Journal of Toxicology and Environmental Health Part A. 74 (17), 1175-1183 (2011).
- Moghe, A., et al. Molecular mechanisms of axrolein toxicity: relevance to human disease. Toxicological Sciences. 143 (2), 242-255 (2015).
- Kreiss, K., Gomaa, A., Kullman, G., Fedan, K., Simoes, E. J., Enright, P. L. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. New England Journal of Medicine. 347 (5), 330-338 (2002).
- Bahl, V., Lin, S., Xu, N., Davis, B., Wang, Y. H., Talbot, P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reproductive Toxicology. 34 (4), 529-537 (2012).
- Gerloff, J., et al. Inflammatory Response and Barrier Dysfunction by Different e-Cigarette Flavoring Chemicals Identified by Gas Chromatography-Mass Spectrometry in e-Liquids and e-Vapors on Human Lung Epithelial Cells and Fibroblasts. Applied In Vitro Toxicology. 3 (1), 28-40 (2017).
- Clapp, P. W., et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. American Journal of Physiology Lung Cell Molecular Physiology. 313 (2), L278-L292 (2017).
- Behar, R. Z., et al. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tobacco Control. 25, ii94-ii102 (2016).
- Pauluhn, J. Overview of inhalation exposure techniques: strengths and weaknesses. Experimental and Toxicologic Pathology. 57 (Suppl 1), 111-128 (2005).
- Oyabu, T., et al. Comparison between whole-body inhalation and nose-only inhalation on the deposition and health effects of nanoparticles. Environmental Health and Preventive. 21 (1), 42-48 (2016).
- Bond, J. A., Chen, B. T., Griffith, W. C., Mauderly, J. L. Inhaled cigarette smoke induces the formation of DNA adducts in lungs of rats. Toxicology and Applied Pharmacology. 99 (1), 161-172 (1989).
- Rudy, A. K., Leventhal, A. M., Goldenson, N. I., Eissenberg, T. Assessing electronic cigarette effects and regulatory impact: challenges with user self-reported device power. Drug and Alcohol Dependence. 179, 337-340 (2017).
- Lee, H. W., et al. E-cigarette smoke damages DNA and reduces repair activity in mouse lung, heart, and bladder as well as in human lung and bladder cells. Proceedings of the National Academy of Sciences (PNAS). , 201718185 (2018).

