Expression of Cementitious Pore Solution and the Analysis of Its Chemical Composition and Resistivity Using X-ray Fluorescence
Summary
This protocol describes the procedure to express fresh pore solution from cementitious systems and the measurement of its ionic composition using X-ray fluorescence. The ionic composition can be used to calculate pore solution electrical resistivity, which can be used, together with concrete electrical resistivity, to determine the formation factor.
Abstract
The goal of this method is to determine the chemical composition and electrical resistivity of cementitious pore solution expressed from a fresh paste sample. The pore solution is expressed from a fresh paste sample using a pressurized nitrogen gas system. The pore solution is then immediately transferred to a syringe to minimize evaporation and carbonation. After that, assembled testing containers are used for the X-ray fluorescence (XRF) measurement. These containers consist of two concentric plastic cylinders and a polypropylene film which seals one of the two open sides. The pore solution is added into the container immediately prior to the XRF measurement. The XRF is calibrated to detect the main ionic species in the pore solution, in particular, sodium (Na+), potassium (K+), calcium (Ca2+), and sulfide (S2-), to calculate sulfate (SO42-) using stoichiometry. The hydroxides (OH–) can be calculated from a charge balance. To calculate the electrical resistivity of the solution, the concentrations of the main ionic species and a model by Snyder et al. are used. The electrical resistivity of the pore solution can be used, along with the electrical resistivity of concrete, to determine the formation factor of concrete. XRF is a potential alternative to current methods to determine the composition of pore solution, which can provide benefits in terms of reduction in time and costs.
Introduction
The transport properties of concrete are determined by its formation factor, which is a fundamental measure of the microstructure1. The formation factor is defined as the inverse of the product between the connectivity and the porosity of a concrete2. The formation factor can be calculated from the ratio of the electrical resistivity of concrete and the electrical resistivity of pore solution as presented in equation 13.
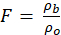 (1)
(1)
Here,
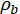 = electrical resistivity of bulk or concrete (Ωm);
= electrical resistivity of bulk or concrete (Ωm);
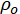 = electrical resistivity of pore solution (Ωm).
= electrical resistivity of pore solution (Ωm).
The bulk electrical resistivity of concrete may be easily determined on hardened concrete using a resistivity meter, following approaches outlined in AASHTO PP84-17 Appendix X2 and other literature4,5. The purpose of this article is to provide instructions for expressing the pore solution from fresh paste and analysis of the solution ionic composition using X-ray fluorescence (XRF) spectroscopy. The expressed pore solution is tested in the XRF using commercially available materials (cylinders and film). The ionic composition detected by the XRF can be used for multiple concrete durability applications and can also be used to calculate the electrical resistivity of pore solution, to ultimately determine the formation factor6.
Current methods to determine the chemical composition of pore solution, such as inductively coupled plasma (ICP)7, atomic absorption spectroscopy (AAS)8, and ion chromatography (IC)9, can be costly, time-consuming, and quite laborious. Additionally, in some cases, a combination of various methods must be used in order to obtain a complete characterization of the main ionic species in pore solution10. XRF can be used as an alternative to these methods, where the composition of pore solution can be obtained at a relatively lower cost and shorter testing time compared to conventional methods.
XRF is a technique commonly used in the cement industry as it is primarily used to analyze the chemical composition of the manufactured materials for quality control and quality assurance throughout the cement manufacturing process11,12. Therefore, this method will describe how that technique can be used to enable cement manufacturers to use this tool to provide more information about the pore solution composition of different cement batches. Overall, using XRF for pore solutions could potentially extend the use of this technique for multiple applications and could be implemented in the industry relatively quickly.
Protocol
1. Pore Solution Expression13
- Make sure the individual components of the pore solution extractor are clean and dry.
- Use a new cellulose filter (with an average pore diameter of 0.45 µm) for each expression.
- Assemble the pore solution extractor, as shown in Figure 1.
- Check that there are no visible deformations in the cellulose filter.
- Add the fresh cementitious paste into the main chamber, leaving it empty for at least 1 cm from the top.
NOTE: The term fresh paste indicates any cementitious paste still in a plastic state. Cementitious pastes are generally made by mixing cement, supplementary cementitious materials, water, and chemical admixtures. The volume ratios of these constituents can vary depending on the properties desired. - Connect the pore solution extractor to the nitrogen source and seal the main chamber.
- Align the expression device with the plastic canister to temporarily collect the extracted pore solution.
- Open the valve of the nitrogen tank and regulate the pressure using the pressure regulator, so that a pressure of approximately 200 kPa is applied to the paste inside the main chamber.
NOTE: For safety, a pressure regulator must be utilized. - Maintain constant pressure for a period of 5 min, during which the pore solution will be collected in the plastic canister.
- After 5 min from the start of the expression, close the main valve so that the pressure inside the main chamber drops to atmospheric pressure.
- Remove the canister from under the extractor and transfer the pore solution to a 5-mL syringe, making sure not to suck in any air bubbles in the process.
- Seal the syringe with its needle cap and move it inside a 5 ± 1 °C chamber to be stored till the time of testing.
- Wait until the pressure gauge shows that there is no additional pressure inside the main chamber and, then, disassemble the pore solution extractor.
- Clean the pore solution extractor parts using deionized water and paper towels.
- Discard the cellulose filter.
2. Assembly of the Solution Containers
- Make sure that the plastic cylinders are clean and dry.
- Place the polypropylene film (commercially available with 0.4-µm thickness, 90 mm in diameter) flat on top of the larger cylinder (commercially available with a 35-mm diameter).
- Insert the smaller cylinder (commercially available with a 32-mm diameter) completely on top of the larger cylinder, pushing down and pressing the film in-between both cylinders to create a plastic container with a polypropylene film base.
- Ensure that the film is smooth and has no tears or deformations.
3. XRF Application Development and Solution Calibration
- Create an application file on the XRF software. The application has to be for solution samples and has to be able to detect the main ionic species in pore solution: sodium (Na+), potassium (K+), calcium (Ca2+), and sulfide (S2-).
- Calibrate the solution application with solutions of known concentrations.
- Prepare the standard solutions using varying concentrations of > 99% pure sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), and aluminum sulfate (Al2[SO4]3) to accurately quantify the element studied.
NOTE: The concentrations of the standards can vary depending on the materials of interest. As an example, it has been observed that the concentrations of Na+ varied between 0 and 0.5 mol/L, the concentrations of K+ between 0 and 0.9 M, the concentrations of Ca2+ between 0 and 0.05 M, and the concentrations of S2- between 0 and 0.25 M; however, exceptions that exceed these limits might occur depending on the system14. The elements defined and measured in the calibration of the application must include all elements used in the calibration standards: sodium (Na+), potassium (K+), calcium (Ca2+), sulfide (S2-), calcium (Cl–), and aluminum (Al3+). - For each calibration solution, measure 6 g of that solution inside the assembled testing container.
- Seal the container with the corresponding lid.
- Leave the testing container with the standard solution on a paper towel for 2 min to ensure that the film has no leaks that could potentially damage the XRF device.
- Place the sealed testing containers with the standard solutions inside the XRF sample holders and close the XRF.
- Measure each standard solution using the XRF. The intensities of characteristic fluorescent X-rays of elements from each of the solutions, measured in counts per minute (cpm), are detected by the XRF.
NOTE: Varying conditions sets for different groups of elements are needed. Refer to a previously published article for parameters such as measuring time and excitation energies6. - Note the concentration in parts per million (ppm) of each element in each standard solution as defined in the software and related to the intensity in counts per minute (cpm) measured by the XRF.
- After the standard solutions are measured, use a matrix correction model from the XRF software used (linear, alphas, fundamental parameters (FP)) that will yield the minimum relative RMS (%) for each element in the calibration to create the best linear fit for the calibration.
- Verify that the application yields accurate results by testing solutions of known concentrations of sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca[OH]2), and aluminum sulfate (Al2[SO4]3) at different concentration levels within the calibration range.
NOTE: The application should yield accurate results if the error is within 5%.
- Prepare the standard solutions using varying concentrations of > 99% pure sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), and aluminum sulfate (Al2[SO4]3) to accurately quantify the element studied.
4. XRF Analysis
- Inject at least 2 g of the pore solution sample in the assembled testing container.
- Seal the container with the corresponding lid.
- Leave the container with the solution on a paper towel for 2 min to ensure that the film has no leaks that could potentially damage the XRF device.
- Place the testing containers with the solutions inside the XRF sample holders and close the XRF.
- On the XRF software, select the XRF application that was previously developed.
- Use the application interface on the software to select the XRF sample holders that are going to be subjected to the X-ray fluorescence analysis.
NOTE: It is recommended to name the new file for each selected sample holder based on the solution being tested. - Start the XRF application to measure the ionic concentrations of the solutions.
NOTE: The results from the XRF analysis will show the concentration of sodium (Na+), potassium (K+), calcium (Ca2+), and sulfide (S2-).
5. Ionic Concentration Calculation
- Use stoichiometry to calculate the concentration of sulfate (SO42-) using equation 2.
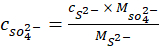 (2)
(2)
Here,
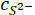 = the measured ionic concentration of sulfide ions from XRF in ppm;
= the measured ionic concentration of sulfide ions from XRF in ppm;
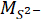 = the molecular weight of sulfide in g/mol;
= the molecular weight of sulfide in g/mol;
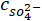 = the measured ionic concentration of sulfate ions from XRF in ppm;
= the measured ionic concentration of sulfate ions from XRF in ppm;
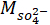 = the molecular weight of sulfate in g/mol.
= the molecular weight of sulfate in g/mol. - Use a charge balance to calculate the concentration of hydroxides (OH–) using equation 3.
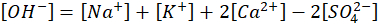 (3)
(3)
Here,
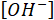 = the hydroxide ions concentration in ppm;
= the hydroxide ions concentration in ppm;
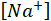 = the sodium ions concentration in ppm;
= the sodium ions concentration in ppm;
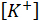 = the potassium ions concentration in ppm;
= the potassium ions concentration in ppm;
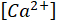 = the calcium ions concentration in ppm;
= the calcium ions concentration in ppm;
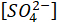 = the sulfate ions concentration in ppm.
= the sulfate ions concentration in ppm. - Convert the ionic concentrations from ppm to mol/L using equation 4 and assuming a density (ρ) of 1,000 g/L. If desired, more accurate density information may be obtained from textbooks15 or thermodynamic software and used.
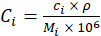 (4)
(4)
Here,
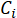 = the ionic concentration of a single ionic species in mol/L;
= the ionic concentration of a single ionic species in mol/L;
 = the ionic concentration of a single ionic species in ppm obtained from XRF;
= the ionic concentration of a single ionic species in ppm obtained from XRF;
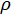 = the density of the solution in g/L;
= the density of the solution in g/L;
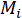 = the molecular weight of a single ionic species in g/mol;
= the molecular weight of a single ionic species in g/mol;
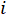 = a single ionic species.
= a single ionic species.
6. Resistivity Calculation
- Use the model developed by Snyder et al.16, expressed in equations 5 – 7, to calculate the electrical resistivity of the pore solution.
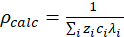 (5)
(5)
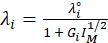 (6)
(6)
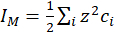 (7)
(7)
Here,
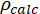 = electrical resistivity of the solution in Ωm;
= electrical resistivity of the solution in Ωm;
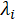 = the equivalent conductivity of a single ionic species in cm2 S/mol;
= the equivalent conductivity of a single ionic species in cm2 S/mol;
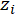 = the valence concentration of a single ionic species;
= the valence concentration of a single ionic species;
 = the molar concentration of a single ionic species in mol/L;
= the molar concentration of a single ionic species in mol/L;
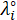 * = the equivalent conductivity of ionic species at infinite dilution in cm2 S/mol;
* = the equivalent conductivity of ionic species at infinite dilution in cm2 S/mol;
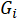 * = the empirical conductivity coefficient of a single ionic species in (mol/L)-1/2;
* = the empirical conductivity coefficient of a single ionic species in (mol/L)-1/2;
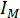 = the ionic strength (molar basis) in mol/L;
= the ionic strength (molar basis) in mol/L;
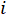 = a single ionic species.
= a single ionic species.
Empirical values can be found in Table 1.
NOTE: The formation factor can then be estimated as the ratio of the electrical resistivity of concrete and the electrical resistivity of pore solution (equation 1)3. As the formation factor is a fundamental descriptor of the concrete microstructure, the determination of the formation factor is an important step in moving a traditionally prescriptive industry toward performance-based specifications. The formation factor has been linked to various transportation phenomena, such as diffusion, absorption, and permeability, and could be used to predict concrete service life1,2,4,5,17,18.
Representative Results
In this section, representative outcomes of each major step in the methodology are presented. This is done in order to obtain an idea of what is expected at the end of each step and provide useful tips to ensure a correct application of the method.
The first important step consists in the expression of the pore solution from the fresh paste sample. Figure 2 shows a pore solution that is correctly extracted and sealed in a 5-mL syringe. The pore solution in the figure was expressed from a fresh ordinary Portland cement paste with a water-to-cement ratio of 0.36. The sample was mixed 10 min before the image was taken. The pore solution is expected to be clear; however, the color can vary depending on the type of cementitious materials that were used and the age of the sample at the time of the expression.
Before the XRF measurement of the extracted pore solution, it is necessary to calibrate the instrument. In particular, each element whose ionic concentration will be measured needs to be calibrated. A representative calibration plot of the potassium (K+) ions is shown in Figure 3. The figure shows the fitting performed by the software on the intensities measured by the XRF. Note that the root mean square (RMS) error of the fitting should stay below 5%.
After calibration, it is recommended to test a solution of known ionic concentration to determine the accuracy of the machine. The measured composition of the ions using XRF is compared to the theoretical composition of both solutions. According to our experience, assuming a correct preparation of the ionic solutions, this checking step should yield a percentage of errors lower than ± 5%. Figure 4 shows the composition results for the spot-checking of the solutions. When the spot-checking yields a percentage of errors higher than ± 5%, repeat the calibration of the XRF device.
Table 2 shows a representative set of results for composition and resistivity. While the ionic concentration of the pore solution can vary widely depending on the chemical composition of the cement, the water-to-cement ratio of the system, and the presence of supplementary cementitious materials19, reference values can be obtained from the literature20 for the main ions, as shown in Table 1.
Finally, when calculating the resistivity of a sample, values for early age pore solutions are typically expected to be within 0.05 and 0.25 Ωm14. Now that the resistivity of the pore solution is known, the bulk resistivity can be obtained using other methods, like uniaxial resistivity, in order to, ultimately, calculate the formation factor, which is typically over 2,000 for good quality concrete4,5,18.
Figure 1: Assembly of the pore solution extraction system. The system consists of a main expression device, a nitrogen tank and tube with a safety pressure gauge and regulator, and a collection container. Always refer to the manufacturer's instructions and safety precautions for the specific system used. Please click here to view a larger version of this figure.

Figure 2: Correctly extracted and sealed extracted pore solution in a 5-mL syringe. The extracted pore solution should appear clear (i.e., no visible particles) and should be sealed with no air bubbles within the syringe.
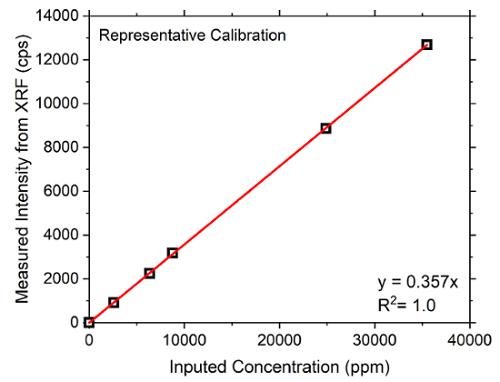
Figure 3: Representative calibration plot of potassium (K+). The x-axis shows the imputed (known) concentrations in ppm, and the y-axis shows the detected (measured) intensities with XRF in cpm. The calibration line calculated from one of the correction models in the software should have the smallest RMS (%), as discussed in section 3 of the protocol. Please click here to view a larger version of this figure.
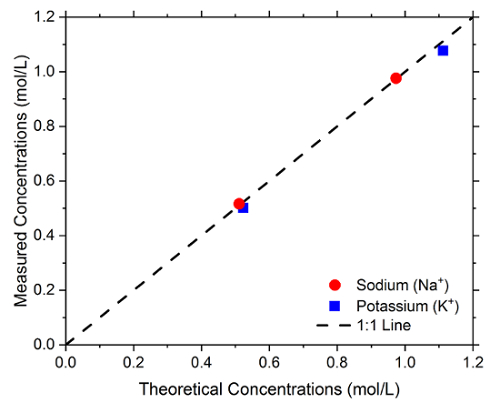
Figure 4: Sodium ion (Na+) and potassium ion (K+) verification plot. The dashed line represents a 1:1 ratio.The verification plot should show a good correlation (almost a 1:1 relationship with a high R-squared value) between the known concentrations of the sodium and potassium ions and the detected concentrations using XRF. Please click here to view a larger version of this figure.
| Ionic Species (i) | Equivalent conductivity at infinite dilution (λ˚i) | Empirical conductivity coefficient |
| (i) | (zλ°i) | (Gi) |
| (cm2 S/mol) | (mol/L)-1/2 | |
| Sodium (Na+) | 50.1 | 0.733 |
| Potassium (K+) | 73.5 | 0.548 |
| Calcium (Ca2+) | 59 | 0.771 |
| Hydroxide (OH–) | 198 | 0.353 |
| Sulfate (SO42-) | 79 | 0.877 |
Table 1: Equivalent conductivity at infinite dilution (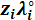 ) and empirical conductivity coefficients (
) and empirical conductivity coefficients (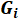 ) for each ionic species obtained from the literature11. These values are used in order to calculate the electrical resistivity of the pore solution.
) for each ionic species obtained from the literature11. These values are used in order to calculate the electrical resistivity of the pore solution.
| Ionic Species | Concentration |
| (i) | (mol/L) |
| Sodium (Na+) | 0.16 |
| Potassium (K+) | 0.39 |
| Calcium (Ca2+) | 0.02 |
| Hydroxide (OH–) | 0.18 |
| Sulfate (SO42-) | 0.2 |
| Resistivity (Ωm) | 0.156 |
Table 2: Representative results for the composition and resistivity of a cement paste with a water-to-cement ratio of 0.36 at 10 min. The values in this table are examples of the results obtained using this method.
Discussion
Since this is a sensitive chemical analysis method, it is imperative to have laboratory practices that prevent contamination. For this method, it is critical that the calibration standards are specifically performed with high-purity chemicals (> 99%). When transferring the pore solution into the syringe, make sure that no visible cement grains are present in the solution to avoid any changes in the pore solution. When stored in a sealed syringe at a constant temperature of 5 ± 1 °C, the pore solution has been observed to maintain an unaltered chemical composition for up to 7 days.
One of the main limitations of this protocol is that the method of expression outlined can only be used for fresh paste specimens and is not suitable for later age samples. For later age or hardened samples, a method of expression using a high-pressure extraction die20 is needed. Another limitation is that a minimum amount of 2 g of solution is needed to test in the XRF since an amount less than 2 g does not provide a constant sample height that can cover the entire bottom face of the container. This last limitation applies to the particular set-up that was used in this study. A different set-up would probably allow a reduction in the minimum amount of pore solution required for the testing. Another limitation is that the model is not likely applicable to systems containing slag-rich cements since species such as bisulfide (HS–) may be present, as discussed by Vollpracht et al.14.
Since XRF is a commonly used technique in the cement industry, this method could potentially enable cement manufacturers to use a tool already at their disposal to provide more information about the cementitious pore solution, such as the chemical composition and resistivity for numerous applications and at a lower cost and testing time than conventional methods. For example, when comparing sample preparation and testing time between ICP (a commonly used testing method for pore solution composition), the testing time is reduced from 50 min per sample to 8 min per sample using XRF. This method could extend the applications for XRF and could potentially be implemented fairly quickly in the industry.
XRF can be used to determine the main elemental concentrations in the pore solution. This suggests the use of XRF for applications such as (i) determining the composition of pore solutions to study the dissolution kinetics of cementitious phases21 or (ii) determining the effect of chemical admixtures22. Early age pore solution and concrete resistivity measurements could be used as a measure of the water-to-cement ratio of concrete, which could potentially be used in quality control.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge partial financial support from the Kiewit Transportation Institute and the Federal Highway Administration (FHWA) through DTFH61-12-H-00010. All of the laboratory work presented herein was performed at the Kiewit Transportation Institute at Oregon State University.
Materials
| Energy Disperssive X-Ray Fluorescence Benchtop Spectrometer | Malvern PANalytical | Epsilon 3XLE or Epsilon 4 | |
| 35 mm Sample Cups for Liquids | Malvern PANalytical | 9425 888 00024 | Panalytical Consumables Catalogue 2016 for XRF Accessories and Consumables Catalog |
| 4 micron Polypropylene Film | Malvern PANalytical | 9425 888 00029 | Panalytical Consumables Catalogue 2016 for XRF Accessories and Consumables Catalog |
| Syringe, 5 mL | VWR | 53548-005 | HSW Norm-Ject Sterile Luer-Slip syringes, Air-Tite |
| Needle, 16Gx1'' | VWR | 89219-334 | Premium Veterinary Hypodermic Needles, Sterile, Air-Tite |
| Container | VWR | 15704-092 | VWR Specimen containers, Polypropylene with Polyethylene Caps |
| Pressurized Filter Holder | EMD Millipore | XX4004700 | 100 mL capacity, 47 mm filter diameter |
| MCE Membrane Filter | PALL | 63069 | 47 mm diameter, 0.45 μm pore size |
| Silicone Funnell | SpiceLuxe | SLP-122513-F1 | Top opening 2 1/2″, Bottom opening 3/4″, Height 2 3/4″ |
References
- Snyder, K. A. Relationship between the formation factor and the diffusion coefficient of porous materials saturated with concentrated electrolytes: theoretical and experimental considerations. Concrete Science and Engineering. 3, 216-224 (2001).
- Dullien, F. . Porous Media: Fluid Transport and Pore Structure. , (1992).
- Archie, G. E. The electrical resistivity log as an aid in determining some reservoir characteristics. Society of Petroleum Engineers. 142 (1), 54-62 (1942).
- Spragg, R., et al. Factors that influence electrical resistivity measurements in cementitious systems. Transportation Research Record: Journal of the Transportation Research Board. 2342, 90-98 (2013).
- Spragg, R. P., Bu, Y., Snyder, K. A., Bentz, D. P., Weiss, J. Electrical Testing of Cement-Based Materials: Role of Testing Techniques, Sample Conditioning, and Accelerated Curing. Joint Transportation Research Program Technical Report. , (2013).
- Tsui-Chang, M., Suraneni, P., Isgor, O. B., Trejo, D., Weiss, W. J. Using X-ray fluorescence to assess the chemical composition and resistivity of simulated cementitious pore solutions. International Journal of Advances in Engineering Sciences and Applied Mathematics. 9 (3), 136-143 (2017).
- Caruso, F., Mantellato, S., Palacios, M., Flatt, R. ICP-OES method for the characterization of cement pore solutions and their modification by polycarboxylate-based superplasticizers. Cement and Concrete Research. 38, 52-60 (2016).
- Capacho-Delgado, L., Manning, D. C. The determination by atomic-absorption spectroscopy of several elements, including silicon, aluminum, and titanium, in cement. Analyst. 92, 552-557 (1967).
- Zanella, R., Primel, E. G., Martins, A. F. Determination of chloride and sulfate in pore solutions of concrete by ion chromatography. Journal of Separation Science. 24 (3), 230-231 (2001).
- Puertas, F., Fernandez-Jimenez, A. Mineralogical and microstructural characterisation of alkali activated fly ash/slag pastes. Cement and Concrete Composites. 25 (3), 287-292 (2003).
- Bouchard, M., et al. Global cement and raw materials fusion/XRF analytical solution II. Powder Diffraction. 26 (2), 176-185 (2011).
- Klockenkamper, R., Bohlen, A. . Total-reflection X-ray Fluorescence Analysis and Related Methods. , (2014).
- Penko, M. . Some early hydration processes in cement pastes as monitored by liquid phase composition measurements. , (1983).
- Vollpracht, A., Lothenbach, B., Snellings, R., Haufe, J. The pore solution of blended cements: a review. Materials and Structures. 49 (8), 3341-3367 (2016).
- Rumble, J. R. . CRC Handbook of Chemistry and Physics. , (2018).
- Snyder, K. A., Feng, X., Keen, B. D., Mason, T. O. Estimating the electrical conductivity of cement paste pore solutions from OH-, K+ and Na+ concentrations. Cement and Concrete Research. 33 (6), 793-798 (2003).
- Weiss, J. Relating transport properties to performance in concrete pavements. CP Road MAP. , (2014).
- Weiss, W. J., Spragg, R., Isgor, O. B., Ley, T. M., Van Dam, T., Hordijk, D. A., Lukovic, M. Toward Performance Specifications for Concrete: Linking Resistivity, RCPT and Diffusion Predictions Using the Formation Factor for Use in Specifications. , 2057-2065 (2017).
- Andersson, K., Allard, B., Bengtsson, M., Magnusson, B. Chemical composition of cement pore solutions. Cement and Concrete Research. 19 (3), 327-322 (1989).
- Barneyback, R., Diamond, S. Expression and analysis of pore fluids from hardened cement pastes and mortars. Cement and Concrete Research. 11 (2), 279-285 (1981).
- Nicoleau, L., Schreiner, E., Nonat, A. Ion-specific effects influencing the dissolution of tricalcium silicate. Cement and Concrete Research. 59, 118-138 (2014).
- Rajabipour, F., Sant, G., Weiss, W. J. Interactions between shrinkage reducing admixtures (SRA) and cement paste’s pore solution. Cement and Concrete Research. 38 (5), 606-615 (2008).

