CO2 Photoreduction to CH4 Performance Under Concentrating Solar Light
Summary
We present a protocol for improving the performance of CO2 photoreduction to CH4 by heightening the incident light intensity via concentrating solar energy technology.
Abstract
We demonstrate a method for the enhancement of CO2 photoreduction. As the driving force of a photocatalytic reaction is from solar light, the basic idea is to use concentration technology to raise the incident solar light intensity. Concentrating a large-area light onto a small area cannot only increase light intensity, but also reduce the catalyst amount, as well as the reactor volume, and increase the surface temperature. The concentration of light can be realized by different devices. In this manuscript, it is realized by a Fresnel lens. The light penetrates the lens and is concentrated on a disc-shaped catalyst. The results show that both the reaction rate and the total yield are efficiently increased. The method can be applied to most CO2 photoreduction catalysts, as well as to similar reactions with a low reaction rate at natural light.
Introduction
The utilization of fossil fuels is accompanied by large amounts of CO2 emission, contributing greatly to global warming. CO2 capture, storage, and conversion are essential to reduce the CO2 content in the atmosphere1. The photoreduction of CO2 to hydrocarbons can reduce CO2, convert CO2 to fuels, and save solar energy. However, CO2 is an extremely stable molecule. Its C=O bond possesses a higher dissociation energy (about 750 kJ/mol)2. This means that CO2 is very hard to be activated and transformed, and only short wavelength lights with high energy can be functional during the process. Therefore, CO2 photoreduction studies suffer from low conversion efficiencies and reaction rates at present. Most reported CH4 yield rates are only at several µmol·gcata-1·h-1 levels on a TiO2 catalyst3,4. The design and fabrication of photocatalytic systems with high conversion efficiency and reaction rate for CO2 reduction remain a challenge.
One popular area of research into CO2 photoreduction catalysts is to broaden the available light band to the visible spectrum and enhance the utilization efficiency of these wavelengths5,6. Instead, in this manuscript, we try to increase the reaction rate by enhancing the light intensity. As the driving force of a photocatalytic reaction is solar light, the basic idea is to use concentration technology to raise the incident solar light intensity and, therefore, increase the reaction rate. This is similar to a thermocatalytic process, where the reaction rate can be increased by increasing the temperature. Of course, the temperature effect cannot be not increased infinitely, and likewise with the light intensity; a major goal of this research is to find a suitable light intensity or concentration ratio.
This is not the first experiment that uses concentrating technology. In fact, it has been widely used in concentrating solar power and waste water treatment7,8. Biomaterials such as beech wood sawdust can be pyrolyzed in a solar reactor9,10. Some previous reports have mentioned the method for CO2 photoreduction11,12,13. One sample exhibited a 50% increment in the product yield when the light intensity was doubled14. Our group has found that concentrating light can raise CH4 yield rate with an up to 12-fold increase in intensity. In addition, pretreatment of catalyst before reaction by concentrating light can further increase the CH4 yield rate15. Here, we demonstrate the experimental system and method in detail.
Protocol
Caution: Please consult all relevant Material Safety Data Sheets (MSDS) before operation. Several chemicals are flammable and highly corrosive. Concentrating light can cause harmful light intensity and temperature increases. Please use all appropriate safety devices such as personal protective equipment (safety glasses, gloves, lab coats, pants, etc.).
1. Catalyst Preparation
- Preparation of TiO2 by anodization
Note: Anodization uses metal foils and a Pt foil as two counter electrodes. The two electrodes are put into the electrolyte. Using electricity, the metal foils at the anode site are oxidized.- Dissolve 0.3 g of NH4F and 2 mL of H2O into 100 mL of glycol in a 200-mL beaker with a stirrer to form the electrolyte. Put the beaker with the electrolyte into a 45 °C water bath.
- Trim the Ti foil (50 x 250 mm size) with scissors to 25 x 25 mm.
- Polish the Ti foil surface with a 7,000-mesh sandpaper to remove the surface impurities.
- Submerge the Ti foil in a volumetric flask containing 15 mL of ethanol, then a flask with 15 mL of acetone, then treat it for 15 min with an ultrasonic cleaner. Take out the Ti foil, rinse it 3 – 5x with deionized water, and place it in a volumetric flask containing 20 mL of ethanol.
- Dissolve 10 mL of H2O, 5 mL of HNO3, 3 mL of H2O2, 1 mL of 18% wt (NH2)2CO, and 1 mL of 18% wt NH4F into a 100-mL beaker to form a polishing solution.
- Take out the Ti foil from the ethanol flask, rinse it 3x with deionized water, and put it into the polishing solution for 2 – 3 min. Remove the Ti foil and wash it with deionized water for 3x.
- Use an anode alligator clip to hold the pretreated Ti foil and another clip to hold a Pt foil (25 x 25 mm). Place the two foils face to face in the electrolyte at a distance of 2 cm from each other. Turn on the direct-current (DC) stabilized current power source, tune the voltage to 50 V, and electrolyze for 30 min.
- After the anodization has finished, close the power and take out the TiO2 foil
- Submerge the Ti foil in a volumetric flask containing 15 mL of ethanol, then a flask with 15 mL of acetone, then treat it for 15 min with an ultrasonic cleaner. Take out the Ti foil, rinse it 3 – 5x with deionized water, and place it in a 50-mL crucible.
- Put the crucible in an oven at 60 °C for 12 h to let the foil dry.
- Calcine the TiO2 foil in a muffle furnace under 400 °C for 2 h with a heating rate of 2 °C/min.
2. Catalytic Tests and Product Analysis
- Catalytic tests under concentrating light
- Clean the stainless cylinder-shaped reactor (inner diameter = 5.5 cm, volume = 100 mL) with deionized water then dry it in an oven at 60 °C for 10 min, to ensure no interference from other carbon sources.
- Take out the reactor from oven, add 2 mL H2O, a stirrer, and a catalyst holder (a small shelf that holds the catalyst in the reactor), and put a quartz glass with pores (diameter = 2 cm) on the bottom of the holder and the TiO2 catalyst (diameter = 1 cm) on the center of the quartz glass. Put a thermocouple through an opening on the reactor wall on the catalyst surface. Add a Fresnel lens on the top of the holder and seal the reactor with a quartz glass window.
- Put the reactor on the electromagnetic apparatus. Check the air tightness with nitrogen (N2).
- Feed the CO2 (99.99%) into the reactor through a mass flow controller (MFC) and flush the reactor at least 3x to change the gas in the reactor to CO2.
- Place the Xe lamp 2 cm directly above the reactor, open the Xe lamp power and adjust its current to 15 A, and turn on the magnetic stirrer switch to start the reaction.
- Record the temperature change on the catalyst surface and in the gas.
- Product analysis
- Analyze the product every 1 h using a gas chromatography (GC), which is equipped with a flame-ionized detector (FID) and a capillary column (see Table of Materials) for separation of C1-C6 hydrocarbons.
- Calculate the number of products by the external standard line method. Before quantifying the product, build a standard curve of methane (CH4).
- Catalytic tests under concentrating light with pretreatment
Note: This procedure is similar to 2.1, with differences noted.- Wash reactor as in step 2.1.1.
- Assemble reactor as in step 2.1.2, except without adding H2O.
- Check the air tightness as in step 2.1.3.
- Feed the pretreatment gas (such as air, N2 and H2O) into the reactor through an MFC and exchange the gas three times in succession to make the reactor pure pretreatment gas.
- Adjust the lamp as in step 2.1.5.
- Keep the catalyst under light (10 concentrating ratio) illumination for 1 h in air atmosphere, then turn off the Xe lamp and magnetic stirrer to finish pretreatment.
- Feed the CO2 (99.99%) into the reactor as in step 2.1.4.
- Inject 2 mL H2O into the reactor from the opening of the wall. Open the Xe lamp and magnetic stirrer power to start the reaction as step 2.1.5.
- Record the temperature change as in step 2.1.6.
Representative Results
The original photocatalytic reactor system mainly contains two components, a Xe lamp and a stainless cylinder reactor. For the concentrating light reactor system, we added a Fresnel lens and a catalyst holder, as shown in Figure 1. The Fresnel lens is used to concentrate the light in a smaller area. As the light has been concentrated, the catalyst must be placed in a lit area; therefore, the catalyst is made into disc shape, and a holder is used to hold the catalyst in this area.
When the anodization method was used, a layer of TiO2 nanotube arrays would form on the foil. Figure 2 displays some characterization results. However, more importantly, TiO2 arrays or other semiconductors could stick on the foil for easy cutting into discs of various sizes without breaking.
We have tested the catalytic performance of as-prepared TiO2 and other semiconductors under concentrating light. Figure 3 displays typical results of CH4 yield versus irradiation time under different concentration ratios (the ratio of the area of light source to the area of the catalyst). The reaction rates of methane on different catalysts were significantly improved under the concentrating conditions. In the case of TiO2, the maximum methane production rate reached 34.56 µmol·gcata-1·h-1. In the case of Fe2O3, the maximum methane production rate reached 19.15 µmol·gcata-1·h-1, which is about 18 times the rate under nature light15. If the catalyst is pretreated with suitable gas (air), the methane production rate can be further increased. The effect is considered to be from the change in surface properties, but more research is needed to prove this.
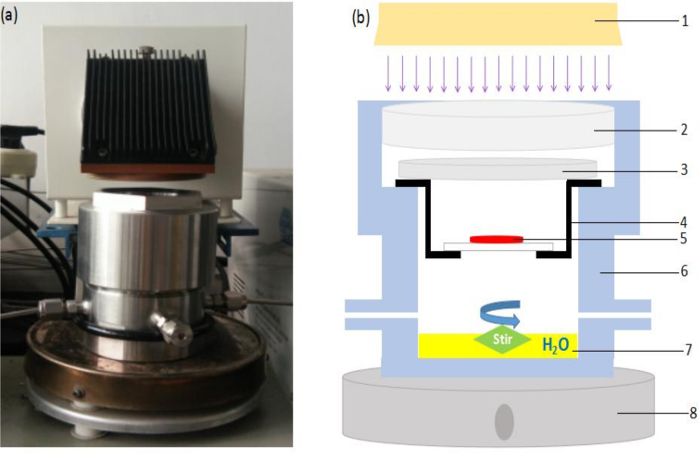
Figure 1: Concentrating light reactor system for the photocatalytic reduction of CO2. (A) Photograph of the set-up. (B) Schematic of the set-up. 1 = Xe lamp, 2 = tquartz glass window, 3 = Fresnel lens, 4 = holder, 5 = photocatalyst, 6 = stainless steel reactor, 7 = H2O, and 8 = magnetic stirrer. Please click here to view a larger version of this figure.
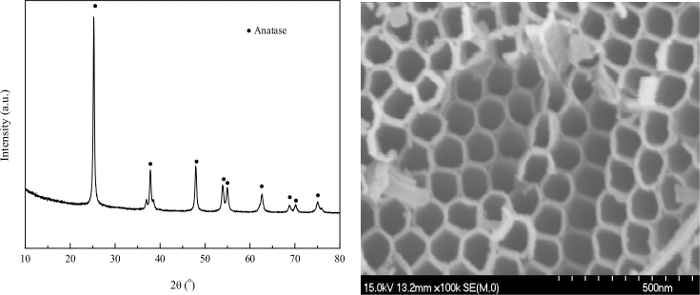
Figure 2: X-ray diffraction (XRD, left) and scanning electron microscope (SEM, right) of TiO2 by anodization. Please click here to view a larger version of this figure.
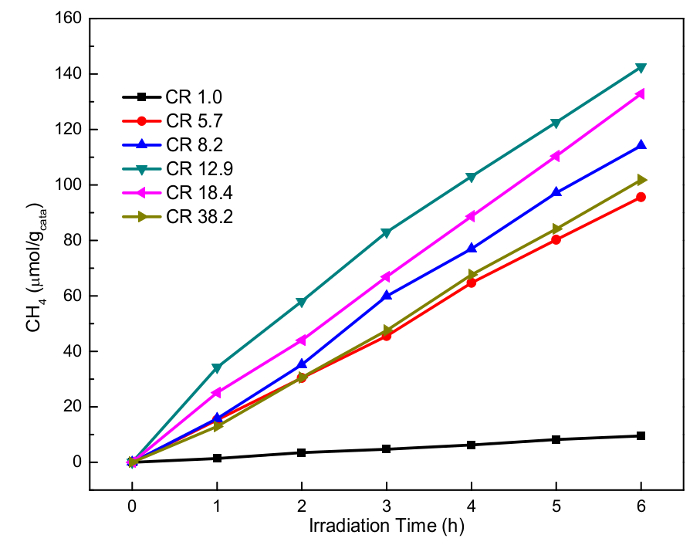
Figure 3: Representative results for the CH4 yield at different concentration ratios (CR). Please click here to view a larger version of this figure.
Discussion
Concentrating light reduces the light incident area and requires the use of a disc-shaped catalyst or a so-called fixed-bed reactor to hold the catalyst. Since the light source is usually a round-shaped lamp, the shape of the catalyst should also be round. To obtain a round disc, it is possible to press the powder into a disk by tableting or to change the metal foil into an oxide by anodization. The anodization method uses electricity to oxidize the metal to an oxide semiconductor. As the metal precursor is already a sheet or foil, it can be trimmed more easily after oxidation without breaking it.
Another factor that needs to be considered is the intensity measurements. We have not given the light intensity after concentration because the use of a commercial detector of light intensity has some limitations. Such a detector often has a large surface area (ID = 1 cm) and a wall to protect it, which will also block much of the light when it is used to measure the concentrating light. Also, when the concentrating ratio is large, the small size of the Xe lamp (which often has an ID of 5 cm) will concentrate the light to a very small area, which may be smaller than the detector area. Therefore, to further investigate the concentrating light technique, large-sized lamps will have to be used and the intensity detector will have to be improved.
After the implementation of the protocol presented here, the CH4 yield rate was clearly enhanced using a suitable concentration ratio, which means that the concentrated light can, to some extent, reduce the amount of catalyst. Of course, a higher light intensity is not always beneficial for a catalytic performance; there is an optimal concentration ratio. Many factors may contribute to the appearance of the optimal concentration ratio. It is known that for the photocatalytic reactions, the reaction order of the light intensity often decreases while the light intensity increases, until it reaches zero. The high intensity also causes the rapid generation and recombination of e–-h+ pairs.
To summarize, we have demonstrated a concentrating light method to improve CO2 photoreduction behavior. Considering the meaning of reducing the catalyst amount and increasing the reaction rate, the method might be useful for the photocatalytic decomposition of H2O, the reduction of CO2, and degradation of volatile organic compounds (VOCs) under real sunlight. At present, there are few studies on photocatalysis under real sunlight, and the yield is very low. Concentration can vastly reduce the volume of the reactor and save costs; in addition, it can increase the light intensity and temperature and, thus, greatly improve the photocatalytic efficiency, but it may be necessary to add an automatic solar tracking system in consideration of the movement of sunlight.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work is supported by the Natural Science Foundation of China (No. 21506194, 21676255).
Materials
| Ti foil, 99.99% | Hebei Metal Technology Co., Ltd. | ||
| Pt foil, 99.99% | Tianjin Aida Henghao Technology Co., Ltd. | ||
| Ammonium fluoride, 98% | Aladdin | A111758 | Humidity sensitive |
| Glycol, >99.9% | Aladdin | E103323 | |
| Anhydrous ethanol,>99.9% | Aladdin | E111977 | Flammable |
| Acetone, >99.5% | Hangzhou Shuanglin Chemical Co., Ltd. | 200-662-2 | Irritating smell |
| Nitric acid, 65.0%-68.0% | Hangzhou Shuanglin Chemical Co., Ltd. | 231-714-2 | Humidity sensitive |
| Hydrogen peroxide, 30 wt. % in H2O | Aladdin | H112515 | Strong oxidative |
| Urea, 99% | Aladdin | U111897 | |
| De-ionized water, 99.00% | Laboratory made | ||
| Xe lamp, CELHXF300/CELHXUV300 | Beijing Zhongjiao Jinyuan Co., Ltd. | ||
| Stainless cylinder reactor, CEL-GPPC | Beijing Zhongjiao Jinyuan Co., Ltd. | ||
| Fresnel lens, MYlens | Meiying Technology Co., Ltd. | ||
| 7000 mesh sandpaper | Zibo Taichuan Abrasives Co., Ltd. | ||
| Ultrasonic cleaner, SK2210HP | Shanghai Kedao Ultrasonic Instrument Co., Ltd. | ||
| Thermostatical water bath, DF-101S | Boncie Instrument Technology Co., Ltd. | ||
| Alligator clip | Guangzhou Rongyu Co., Ltd. | ||
| DC constant voltage source, DY-150V 2A | Shanghai Anding Electric Co., Ltd. | ||
| Muffle furnace, KSL-1200X | Hefei Kejing Materials Technolgy Co., Ltd. | ||
| Quartz glass | Lianyungang Weida Quartz Products Co., Ltd. | ||
| Thermocouples, WRNK-191K | Feiyang Electric Accessories Co., Ltd. | ||
| Electronmagnetic stirrer, 85-2 | Shanghai Zhiwei Electric Appliance Co., Ltd. | ||
| Vacuum pump,SHB-IIIA | Henan Province Taikang science and education equipment factory | ||
| Gas Chromatograph, GC2014 | SHIMAPZU | ||
| HT-PLOT Q capillary column | Hychrom | ||
| Optical power meter,CEL-NP2000 | Beijing Zhongjiao Jinyuan Co., Ltd. | ||
| Electronic scale, JJ124BC | Shanghai Jingtian Electronic Instrument Co., Ltd. |
References
- De-Richter, R. K., Ming, T., Caillol, S. Fighting global warming by photocatalytic reduction of CO2, using giant photocatalytic reactors. Renewable & Sustainable Energy Reviews. 19 (1), 82-106 (2013).
- Fang, Y., Wang, X. Photocatalytic CO2 conversion by polymeric carbon nitrides. Chemical Communications. 54 (45), 5674-5687 (2018).
- Kondratenko, E. V., et al. Status and perspectives of CO2 conversion into fuels and chemicals by catalytic, photocatalytic and electrocatalytic processes. Energy & Environmental Science. 6 (11), 3112-3135 (2013).
- Izumi, Y., Jin, F., He, L. -. N., Hu, Y. H. Recent Advances (2012-2015) in the Photocatalytic Conversion of Carbon Dioxide to Fuels Using Solar Energy: Feasibilty for a New Energy. Advances in CO2 Capture, Sequestration, and Conversion. , 1-46 (2015).
- White, J. L., et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chemical Reviews. 115 (23), 12888-12935 (2015).
- Habisreutinger, S. N., Schmidtmende, L., Stolarczyk, J. K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angewandte Chemie International Edition. 52 (29), 7372-7408 (2013).
- Weinstein, L. A., et al. Concentrating Solar Power. Chemical Reviews. 115 (23), 12797-12838 (2015).
- Herrmann, J. M., et al. TiO2 -based solar photocatalytic detoxification of water containing organic pollutants. Case studies of 2, 4-dichlorophenoxyaceticacid (2, 4 – D) and of benzofuran. Applied Catalysis B Environmental. 17 (1-2), 15-23 (1998).
- Zeng, K., et al. Combined effects of initial water content and heating parameters on solar pyrolysis of beech wood. Energy. 125, 552-561 (2017).
- Zeng, K., et al. Characterization of solar fuels obtained from beech wood solar pyrolysis. Fuel. 188, 285-293 (2017).
- Nguyen, T. V., Wu, J. C. S., Chiou, C. H. Photoreduction of CO over Ruthenium dye-sensitized TiO-based catalysts under concentrated natural sunlight. Catalysis Communications. 9 (10), 2073-2076 (2008).
- Guan, G., et al. Photoreduction of carbon dioxide with water over K2Ti6O13, photocatalyst combined with Cu/ZnO catalyst under concentrated sunlight. Applied Catalysis A: General. 249 (1), 11-18 (2003).
- Han, S., Chen, Y. F., Abanades, S., Zhang, Z. K. Improving photoreduction of CO2 with water to CH4 in a novel concentrated solar reactor. Journal of Energy Chemistry. 26 (4), 743-749 (2017).
- Roy, S. C., et al. Toward solar fuels: photocatalytic conversion of carbon dioxide to hydrocarbons. ACS Nano. 4 (3), 1259-1278 (2010).
- Li, D., Chen, Y. F., Abanades, S., Zhang, Z. K. Enhanced activity of TiO2 by concentrating light for photoreduction of CO2 with H2O to CH4. Catalysis Communications. 113, 6-9 (2018).

