Induction of Mouse Lung Injury by Endotracheal Injection of Bleomycin
Summary
Here we present an effective method to investigate the antifibrotic activity of intravenously infused human mesenchymal stromal cells obtained from the whole umbilical cord following the induction of lung injury by an endotracheal injection of bleomycin in C57BL/6 mice. This protocol can be easily extended to the preclinical testing of other therapeutics.
Abstract
Pulmonary fibrosis is a hallmark of several human lung diseases with a different etiology. Since current therapies are rather limited, mouse models continue to be an essential tool for developing new antifibrotic strategies. Here we provide an effective method to investigate in vivo antifibrotic activity of human mesenchymal stromal cells obtained from whole umbilical cord (hUC-MSC) in attenuating bleomycin-induced lung injury. C57BL/6 mice receive a single endotracheal injection of bleomycin (1.5 U/kg body weight) followed by a double infusion of hUC-MSC (2.5 x 105) into the tail vein, 24 h and 7 days after the bleomycin administration. Upon sacrifice at days 8, 14, or 21, inflammatory and fibrotic changes, collagen content, and hUC-MSC presence in explanted lung tissue are analyzed. The injection of bleomycin into the mouse trachea allows the direct targeting of the lungs, leading to extensive pulmonary inflammation and fibrosis. The systemic administration of a double dose of hUC-MSC results in the early blunting of the bleomycin-induced lung injury. Intravenously infused hUC-MSC are transiently engrafted into the mouse lungs, where they exert their anti-inflammatory and antifibrotic activity. In conclusion, this protocol has been successfully applied for the preclinical testing of hUC-MSC in an experimental mouse model of human pulmonary fibrosis. However, this technique can be easily extended both to study the effect of different endotracheally administered substances on the pathophysiology of the lungs and to validate new anti-inflammatory and antifibrotic systemic therapies.
Introduction
Pulmonary fibrosis is a progressive pathological process characterized by the excessive deposition of extracellular matrix components, mainly type I collagen, in the lung interstitium, leading to impaired lung function. It is the hallmark of several human lung diseases with a different etiology and represents a poor clinical prognostic factor. Since current therapies are rather limited1, mouse models continue to be an essential tool both for the further investigation of the pathogenic mechanisms influencing the onset and the progression of the disease and for developing new antifibrotic strategies2,3.
To date, the administration of bleomycin has been the most commonly applied model of experimentally induced pulmonary fibrosis4. Beside multiple delivering methods (including intravenous, intraperitoneal, subcutaneous, and inhalational), intratracheal or endotracheal injections of bleomycin have emerged as the most frequently used routes4,5. The method that we describe herein has been developed to avoid the scalding effect of bleomycin on the tracheal mucosa. In fact, by exteriorizing the trachea and visualizing it through an operating microscope, it is possible to achieve the instillation of the entire volume of bleomycin solution directly into the lower airway without any spills in the upper airway. When the required surgical expertise and instrumentation are available, this method allows for the safe, robust, and reproducible induction of lung inflammation and fibrosis, as reported below.
Protocol
All animal care and experimental procedures were approved by the Italian Ministry of Health (authorization n. 456/2016-PR) and performed according to the Declaration of Helsinki conventions.
1. Mice
- After purchasing them, allow the mice to acclimate for at least 7 days before the injection.
NOTE: Mice were housed in the animal facility under pathogen-free conditions, were maintained under constant temperature and humidity on a 12 h light/dark cycle, and were given free access to water and standard pellet food. - Use female C57BL/6 mice and inject them at 12 to 16 weeks of age.
2. Endotracheal injection of bleomycin
- Bleomycin preparation
CAUTION: Based on the Globally Harmonized System of Classification and Labelling of Chemicals (GHS), bleomycin is classified as a GHS08 health hazard.- Prepare bleomycin under a chemical hood.
- To obtain the desired working concentration (0.05 U/100 µL), resuspend 15 U of lyophilized bleomycin sulfate in 30 mL of sterile saline.
- Carefully mix the sample by inverting the tube to avoid clot formation.
- Properly label the tube with the date of resuspension, store it at 4 °C, and use its content within 24 h.
- Prior to the instillation, equilibrate the bleomycin solution to room temperature.
NOTE: In this experiment, a single dose of 1.5 U/kg body weight of bleomycin was used to induce lung injury in C57BL/6 mice. Nevertheless, each mouse strain has a different sensitivity to bleomycin6,7. Titration of bleomycin should be performed to determine the optimal dose in the mouse strain used for the experiments.
- Anesthesia
- Prepare anesthesia by dissolving 0.2 g of 2,2,2-tribromoethanol in 9 mL of sterile saline and 1 mL of absolute ethanol (at a working concentration of 20 mg/mL).
- Mix thoroughly by inverting the tube to avoid clot formation.
- Properly label the tube with the date of preparation, store it at 4 °C in darkness, and use it within 3 days.
- Anesthetize the mice with an intraperitoneal injection of 250 µL of tribromoethanol solution (at a final dose of 200 mg/kg body weight) per mouse, using a 1 mL syringe and a 26 G needle.
NOTE: With this dose, the mice are unconscious for at least 20 min. When necessary, adjust the dosage according to the mouse response, in consultation with the veterinarian. - Monitor the mouse breathing. The respiration rate will slightly slow down. After a few minutes, pinch one of the mouse feet to check the lack of pedal reflex.
- Endotracheal injection
- Maintain aseptic conditions during all the procedure. Use sterile surgical instruments and materials, wear sterile gloves and avoid contact with any non-sterile surface.
- Lie down the anesthetized mouse on its back on a surgical platform and hold it in place by delicately fixing its legs with surgical tape strips.
NOTE: Gentle taping of mouse legs is recommended to avoid mouse sliding away from the surgical platform during its rotation (step 2.3.10). - Place the mouse on heated mat to keep the rectal temperature stable at 37 °C throughout the intervention. Measure the rectal temperature by a rectal probe.
- Gently hyperextend the mouse neck by placing a “pillow”, for example, a dental cotton roll, beneath its cervical region.
- Gently shave the throat with a razor blade.
- Remove hair from the surgical area with alcohol and disinfect the mouse skin several times with 1% povidone-iodine solution.
- Pinch the skin with a pair of anatomical forceps and make a short incision in correspondence of the mouse sternohyoid muscle, using a pair of ring-handled, curved blunt scissors.
NOTE: The incision of the skin is about 0.5 cm in length. The corresponding skin piece which is removed is so small that it will create no tension in the mouse neck. - Stop the bleeding with cotton wool sticks.
- Exteriorize the trachea by blunt dissection, gently cleaning it from fat and other tissues.
- Rotate the surgical platform to orient the mouse with its head toward the operator.
NOTE: This position allows the operator, during the injection, to angle the syringe so that it follows the natural path of the trachea straight to the lungs. - Place the mouse under an operating microscope to help with the visualization of the trachea. Adjust the illumination and set the magnification (between 1 and 1.2), focus, and sharpness. The trachea can be easily distinguished as a white translucent tube, and the tracheal rings are clearly visible.
- Mix the bleomycin solution by gently pipetting, and aspirate 100 µL into a 0.5 mL syringe with a 25 G needle, avoiding bubble formation.
- Once the trachea in clearly visualized, carefully puncture it with the needle tip at an angle of 30° (Figure 1A).
- Slowly instill 100 µL of bleomycin or sterile saline (vehicle control) directly into the lumen of the trachea. Wait a few seconds until the entire volume travels down the needle, and then remove it from the trachea.
- Observe a few seconds of apnea, which occurs when the needle is correctly inserted into the trachea so that the mouse will immediately inhale the entire volume of the liquid.
- If the mouse is not inhaling the liquid, carefully monitor its breathing and adjust the needle position. If the mouse stops breathing, immediately remove the needle and allow the mouse to resume breathing normally before reinserting it.
- Safely discard the syringe and needle after the injection.
- Close the subcutaneous fascia and the skin wound with a 5-0 absorbable suture.
NOTE: When not completely reabsorbed, remove sutures 7-10 days post-surgery.
- Animal recovery
- Place the injected mouse on its side on a heating pad for recovery.
- Monitor the mouse breathing and observe the mouse until it starts moving and regains sternal recumbency and full consciousness.
- Once it is confirmed that the mouse is in good condition, return it to the original cage. Do not return it to the company of other animals until it has fully recovered.
- To ensure prolonged analgesia and avoid any residual post-interventional pain, administer subcutaneously buprenorphine (at a final dose of 0.05 mg/kg body weight) to mouse every 12 h post endotracheal injection.
- Examine the mice for 24 h after the endotracheal injection of bleomycin and do so twice a day. Monitor the mice for respiratory distress, weight loss, behavior abnormalities, and for any sign of morbidity.
3. Tail vein infusion of human umbilical cord mesenchymal stromal cells
- Cell preparation
NOTE: The isolation, characterization, and cultivation of mesenchymal stromal cells from human umbilical cord has previously been described8,9,10. hUC-MSC should be aseptically manipulated and infused; therefore, perform all steps under a sterile hood.- Expand the hUC-MSC in 75 cm2 culture flasks to early passages (1–3 maximum).
NOTE: The hUC-MSC should be 70% confluent at the day of their infusion into mice. - Wash the cells with 10 mL of sterile phosphate-buffered saline (PBS) at room temperature.
- Add 2 mL of trypsin and incubate the cells at 37 °C for about 1 min, until they start detaching.
- Neutralize the trypsin by adding 8 mL of hUC-MSC complete medium containing 10% fetal bovine serum (FBS).
- Collect the cells by centrifugation at 350 x g for 10 min.
- Resuspend the pellet in sterile saline and count the cells using a Bürker chamber. Prepare the cell suspension for infusion by diluting the cells to a final concentration of 2.5 x 105 in 200 µL of sterile saline per mouse. Prepare an excess cell suspension to ensure there is enough volume for infusing all mice.
- Keep the cells on ice prior to the infusion. Infuse within a few hours, as described in section 3.3.
- Expand the hUC-MSC in 75 cm2 culture flasks to early passages (1–3 maximum).
- Anesthesia
NOTE: In order to minimize the risk of damaging the mouse tail vein during injection the mouse must not move. Therefore anesthesia has been preferred over simple mouse restraining.- Anesthetize the mice by 4% isoflurane inhalation in an induction chamber.
- Monitor the mouse breathing. The respiration rate will slightly slow down. After a few minutes, pinch one of the mouse feet to check for proper anesthetization.
- Tail vein infusion
- Once unconsciousness has been confirmed, place the mouse under a sterile hood for the aseptic hUC-MSC intravenous infusion.
- Maintain general anesthesia throughout the experiment via a facial mask with a continuous flow of 1.5% isoflurane.
- To promote vasodilation and allow for an easier injection, soak the mouse tail in warm water for 2 min.
- Mix the cell suspension by gently pipetting to make sure that the cells do not form clumps. Aspirate 200 µL into a 1 mL syringe with a 26 G needle, avoiding bubble formation.
- Hold the mouse tail by the tip and gently straighten it.
- Locate the lateral vein of the mouse tail; gently scrape it with a scalpel and wipe it with 70% ethanol.
NOTE: Gently scraping of the tail is performed to remove hair, making the site of injection smoother and cleaner. - Starting from the distal portion of the tail, insert the needle into the vein at a 15° angle and slowly infuse 200 µL of hUC-MSC or sterile saline (vehicle control) (Figure 1B).
- Monitor the successful intravenous infusion by the liquid entering the vein without resistance and by a lack of extravasation. Wait a few seconds until the entire volume travels down the needle, and then remove it from the vein.
- To prevent bleeding, briefly apply pressure to the entry wound with a sterile gauze.
- Safely discard the syringe and the needle after the infusion.
- Animal recovery
- Place the infused mouse on its side on a heating pad for recovery.
- Monitor the mouse breathing and observe the mouse until it starts moving and regains sternal recumbency and full consciousness.
- Once it is confirmed that the mouse is in good condition, return it to the original cage. Do not return it to the company of other animals until it has fully recovered.
- Examine the mice for 24 h after the tail vein infusion and every other day, to monitor their health status and detect any suffering or pathological sign early.
4. Organ explant and tissue processing
- Sacrifice the mice at days 8, 14, or 21 after the bleomycin administration (Figure 1C) by overdose of injectable anesthetic.
- Excise the trachea and the lungs and immediately wash them in ice-cold PBS.
- Snap-freeze the right lungs in liquid nitrogen and store them at -80 °C for a subsequent molecular analysis10.
- Inflate the left lungs with 4% paraformaldehyde and fix them in 10% neutral-buffered formalin solution for 24 h; then, dehydrate them in graded alcohol series, clear them in xylene, and embed them in paraffin10.
Representative Results
Lung injury was induced by a single endotracheal injection of 1.5 U/kg body weight of bleomycin sulfate in 100 µL of sterile saline. Control animals received an endotracheal injection of an equal volume of saline. Two shots of hUC-MSC (2.5 x 105 in 200 µL of sterile saline) were infused into the mouse tail vein, 24 h and 7 days after the bleomycin administration. Control animals received an intravenous infusion of an equal volume of sterile saline. Mice were sacrificed for lung explant and tissue processing at days 8, 14, and 21 after the bleomycin administration (Figure 1).
We demonstrated that a direct instillation of bleomycin into the mouse trachea allowed a rapid diffusion down to the lungs, resulting in extensive inflammation, progressive fibrosis, and a distortion of their normal architecture, consistently with prior studies11. Lung histopathological changes were assessed by hematoxylin-eosin (H&E) and picrosirius red staining10, and fibrosis was confirmed by an increased hydroxyproline content and collagen deposition (Figure 2). Inflammatory changes in tissue were assessed by a histological scoring system based on the inflammatory infiltration around bronchioles, bronchi, and blood vessels, and interstitial pneumonia observed in hematoxylin-eosin stained lung sections10. Following the bleomycin injection, the Ashcroft score of lung sections progressively increased from a mean value of 1.5 at day 8 to a mean value of 4.5 at day 14 and of 6.5 at day 2110. The double infusion of hUC-MSC into the mouse tail vein largely attenuated bleomycin-induced lung injury, with significant reduction, although not complete abrogation, of both the inflammatory infiltration and the extent of fibrosis at each time point (Figure 2). Immunostaining with specific antibodies10 showed that infused hUC-MSC rapidly and effectively reached mouse lungs, although only a few cells were detected, with a decreasing number from day 8 to day 21 (Figure 3). As previously reported12, these data suggest a rapid dislocation of the cells from the site of injury, despite their prolonged protective effect. Immunohistochemistry (IHC) staining of hUC-MSC was performed, also in the saline-treated samples, but no cell could be detected, given the absence of inflammatory foci attracting hUC-MSC.
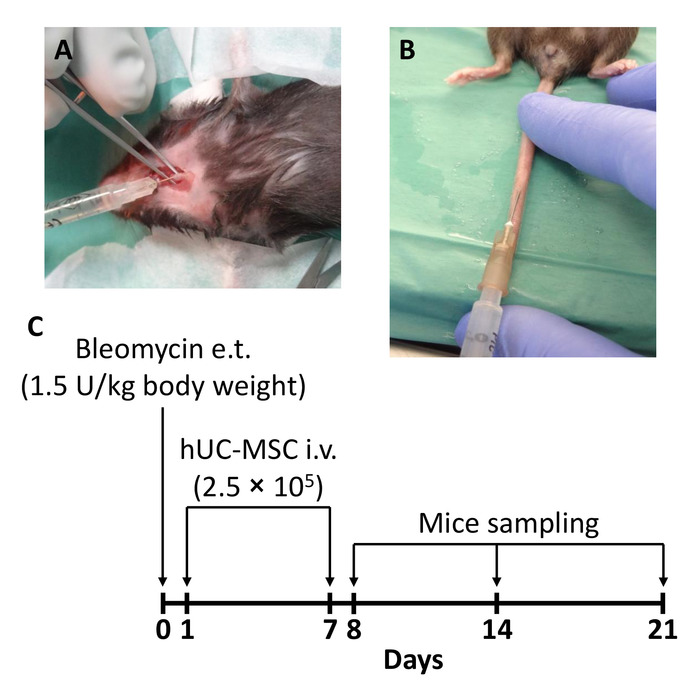
Figure 1: Schematic of the experimental protocol. (A) Mice received a single endotracheal (e.t.) injection of 1.5 U/kg body weight of bleomycin to induce lung injury (day 0). (B) A double intravenous (i.v.) infusion of 2.5 x 105 human mesenchymal stromal cells obtained from whole umbilical cord (hUC-MSC) was performed 24 h (day 1) and 7 days (day 7) after the bleomycin administration. (C) A timeline of the injections and moments of sacrifice is shown here. Mice groups were sacrificed at days 8, 14, and 21 after the bleomycin administration (i.e., 24 h, 7 days, and 14 days after the second hUC-MSC infusion, respectively). This figure has been modified from Moroncini et al.10. Please click here to view a larger version of this figure.
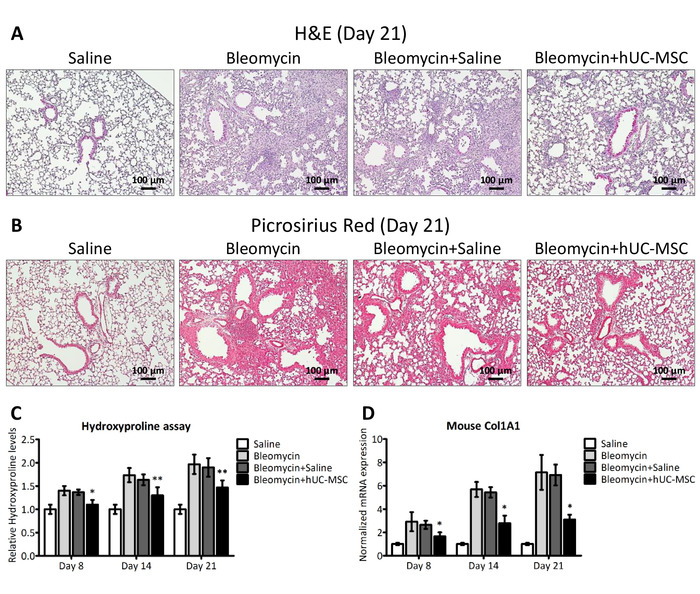
Figure 2: hUC-MSC downregulate bleomycin-induced lung inflammation and fibrosis. (A and B) Representative microscopic images (10x magnification) of hematoxylin-eosin (H&E) and picrosirius red staining of lung sections obtained from C57BL/6 mice, 21 days after the endotracheal injection of sterile saline (saline) or bleomycin (bleomycin), the latter also followed by an intravenous infusion of hUC-MSC (bleomycin+hUC-MSC) or sterile saline (bleomycin+saline). The saline controls demonstrated normal lung architecture. Widespread inflammatory infiltrates were observed 21 days after the bleomycin injury, with a severe distortion of the lung architecture and the formation of fibrotic foci. Bleomycin-induced alterations were significantly attenuated by the hUC-MSC treatment but not by saline. (C) Hydroxyproline content at days 8, 14, and 21 in the lungs of C57BL/6 mice that received the aforementioned treatments. The results are the mean ± SD (n = 8 per group), expressed as a percentage of the value obtained from endotracheal saline-treated mice and are representative of three independent experiments. *P < 0.05, **P < 0.01, compared to bleomycin-treated mice. (D) Mouse Col1A1 expression levels in whole lung mRNA obtained at days 8, 14, and 21 from C57BL/6 mice that received the aforementioned treatments. The results are the mean ± SD (n = 5 per group) and are representative of three independent experiments performed in triplicate. *P < 0.05, **P < 0.01, compared to bleomycin-treated mice. This figure has been modified from Moroncini et al.10. Please click here to view a larger version of this figure.
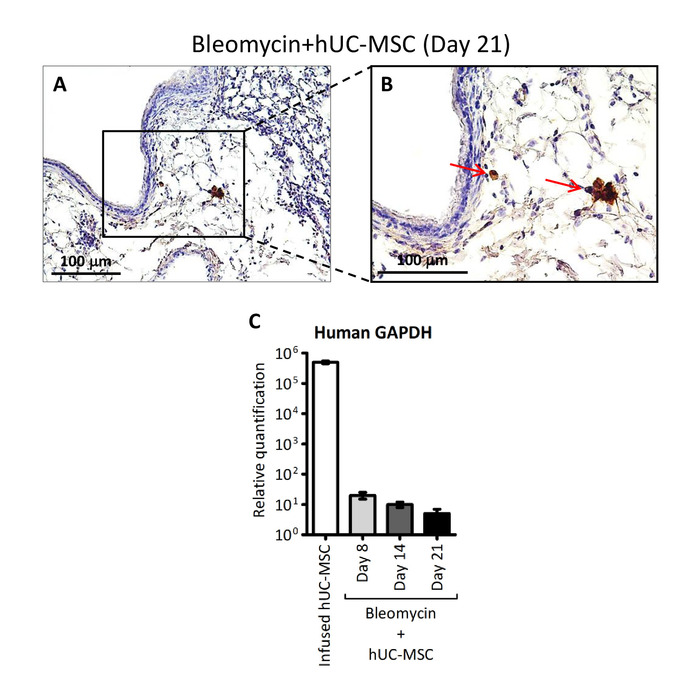
Figure 3: Detection of hUC-MSC in lung tissue. (A and B) Representative microscopic images (200x and 400x magnification, respectively) of immunostaining with anti-HLA-1 antibody of lung sections obtained from C57BL/6 mice receiving endotracheal bleomycin followed by intravenous hUC-MSC. The red arrows indicate positive-stained hUC-MSC. (C) Human GAPDH assessed by quantitative real-time polymerase chain reaction (PCR) assay in whole mRNA extracted from cultured hUC-MSC prior to infusion (infused hUC-MSC) or from lung tissue at days 8, 14, and 21 of C57BL/6 mice receiving endotracheal bleomycin followed by intravenous hUC-MSC (bleomycin+hUC-MSC). The results are the mean ± SD (n = 5 per group) and are representative of three independent experiments performed in triplicate. Of note, the source of human GAPDH transcript in this experimental protocol can be provided exclusively by the intravenously infused hUC-MSC. This figure has been modified from Moroncini et al.10. Please click here to view a larger version of this figure.
Discussion
Endotracheal administration is the preferential route for delivering exogenous agents into the lungs. Since several years, the direct injection of bleomycin into the trachea has been widely used to induce pulmonary fibrosis13 and, recently, more advanced, noninvasive techniques have been developed to accomplish this14,15,16.
The method described here provides several meaningful benefits over some potential limitations. Injection of the trachea requires a surgical intervention, carrying with it the potential for complications caused by the procedure itself, together with the need for deep animal sedation. Therefore, good preparation and practice in perfecting the procedure are needed. Besides, to minimize mouse suffering, it is imperative to set the appropriate dose of anesthetic according to the mouse strain and to the individual response and to maintain a rigorous observation of the animal sedation state. Nevertheless, we observed a very low rate of mortality and optimal animal recovery from anesthesia. Ketamine and xylazine can be used for anesthesia, as well as tribromoethanol. However, in mice, the effective dose of ketamine and xylazine is close to the lethal dose; thus, they can easily induce a respiratory arrest. Conversely, tribromoethanol dosing can be easily adjusted and is, thus, a preferable anesthetic agent. Following the endotracheal injection of bleomycin into the trachea, we did not observe any adverse effects. The mice were free from fever and no signs of inflammation or infection were observed around the trachea and the skin wound. Therefore, there was no need for antibiotic prophylaxis. Moreover, the use of an operating microscope ensures a high confidence of success by allowing the operator to accurately monitor the correct placement of the needle into the mouse trachea prior to the instillation, thus minimizing the risk of damaging it.
The endotracheal injection of bleomycin results in a potent inflammatory and fibrotic response in both lungs and can be seen as a robust method to generate experimental mouse models of human interstitial lung diseases (ILD). However, as previously documented7, the fibrotic response to bleomycin in mice is strain-dependent and gender- and age-related. Therefore, it is critical to the success of the protocol to find the tolerable dose of bleomycin in every experimental setting. Female mice were used in this study because the main interest in this research was interstitial lung disease associated with systemic sclerosis, which is a disease largely prevalent in young adult females. Three- to four-month-old mice were chosen because this is the age at which they just enter the adult phase (mice attain sexual maturity at 8-12 weeks of age)17. Thus, they are considered to be young adult mice and are preferable over younger animals, since lung fibrosis is not common in very young individuals. They are also preferable over older animals since previous studies18 have demonstrated that aged mice exhibited alterations in the lung fibroblast phenotype, leading to an increased susceptibility to disrepair and fibrosis after lung injury, which could represent a possible bias in the experimental model presented here.
Tail vein infusion is a simple, reliable, and noninvasive way to ensure the rapid and effective delivery of drugs to the bloodstream. It can be easily performed with simple medical equipment, short manual training, and reduced costs.
The experimental protocol described here, modified from previously published studies19,20,21, exists of a double intravenous infusion of 2.5 x 105 hUC-MSC to enhance cell engraftment into the mouse lungs and their therapeutic effect. In fact, since the procedure is nontraumatic, it can be repeated in the same animal, but a period of 7 days between two consecutive injections is recommended, to allow the reparation of eventual vasal wounds. Moreover, we used isoflurane inhalation to anesthetize the C57BL/6 mice during the procedure, to avoid tail vein injury in case of sudden animal movements.
In conclusion, this protocol has been successfully applied to efficiently induce pulmonary fibrosis in C57BL/6 mice and to validate the in vivo antifibrotic effect of hUC-MSC. This method can also be used for administering drugs or agents other than bleomycin into the airway, in order to generate different experimental lung disease models.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant RF-2011-02352331 from Ministero Italiano della Salute (to Armando Gabrielli).
Materials
| C57BL/6 mice | Charles River | Jax Mice Stock n. 000664 | |
| 2,2,2-Tribromoethanol (Avertin) | Sigma-Aldrich | T48402 | |
| Barraquer Micro Needle Holder | Lawton | 62-3755 | |
| Bleomycin sulfate | Sigma-Aldrich | B1141000 | |
| Bürker chamber | Brand | 718905 | |
| Culture Flasks | EuroClone | ET7076 | |
| Disposable razors | Unigloves | 4080 | |
| Dissecting Forceps | Aesculap Surgical Instruments | BD311R | |
| DPBS | Gibco | 14190-144 | |
| Heating pad | 2Biological Instruments | 557023 | |
| Isoflurane Vet | Merial Italia | N01AB06 | |
| Operating Microscope | Carl Zeiss | Model OPM 16 | |
| TrypLE Select Enzyme | Gibco | 12563-029 | |
| Vannas Micro Scissors | Aesculap Surgical Instruments | OC498R | |
| Vicryl Plus 4/0 Absorbable Suture, FS-2 needle 19 mm | Ethicon | VCP392ZH |
References
- Iudici, M., et al. Where are we going in the management of interstitial lung disease in patients with systemic sclerosis?. Autoimmunity Reviews. 14 (7), 575-578 (2015).
- Moore, B. B., Hogaboam, C. M. Murine models of pulmonary fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 294 (2), L152-L160 (2008).
- Mouratis, M. A., Aidinis, V. Modeling pulmonary fibrosis with bleomycin. Current Opinion in Pulmonary Medicine. 17 (5), 355-361 (2011).
- Moeller, A., Ask, K., Warburton, D., Gauldie, J., Kolb, M. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis?. International Journal of Biochemistry and Cell Biology. 40 (3), 362-382 (2008).
- Moore, B. B., et al. Animal models of fibrotic lung disease. American Journal of Respiratory Cell and Molecular Biology. 49 (2), 167-179 (2013).
- Schrier, D. J., Kunkel, R. G., Phan, S. H. The role of strain variation in murine bleomycin-induced pulmonary fibrosis. American Review of Respiratory Disease. 127 (1), 63-66 (1983).
- Phan, S. H., Kunkel, S. L. Lung cytokine production in bleomycin-induced pulmonary fibrosis. Experimental Lung Research. 18 (1), 29-43 (1992).
- Capelli, C., et al. Minimally manipulated whole human umbilical cord is a rich source of clinical-grade human mesenchymal stromal cells expanded in human platelet lysate. Cytotherapy. 13 (7), 786-801 (2011).
- Beeravolu, N., et al. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. Journal of Visualized Experiments. (122), e55224 (2017).
- Moroncini, G., et al. Mesenchymal stromal cells from human umbilical cord prevent the development of lung fibrosis in immunocompetent mice. PLoS One. 13 (6), e0196048 (2018).
- Shahzeidi, S., Jeffery, P. K., Laurent, G. J., McAnulty, R. J. Increased type I procollagen mRNA transcripts in the lungs of mice during the development of bleomycin-induced fibrosis. European Respiratory Journal. 7 (11), 1938-1943 (1994).
- Lee, R. H., et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 5 (1), 54-63 (2009).
- Scotton, C. J., Chambers, R. C. Bleomycin revisited: towards a more representative model of IPF?. American Journal of Physiology-Lung Cellular and Molecular Physiology. 299 (4), L439-L441 (2010).
- Cai, Y., Kimura, S. Noninvasive intratracheal intubation to study the pathology and physiology of mouse lung. Journal of Visualized Experiments. (81), e50601 (2013).
- Thomas, J. L., et al. Endotracheal intubation in mice via direct laryngoscopy using an otoscope. Journal of Visualized Experiments. (86), e50269 (2014).
- Vandivort, T. C., An, D., Parks, W. C. An Improved Method for Rapid Intubation of the Trachea in Mice. Journal of Visualized Experiments. (108), e53771 (2016).
- Dutta, S., Sengupta, P. Men and mice: Relating their ages. Life Sciences. 152, 244-248 (2016).
- Sueblinvong, V., et al. Predisposition for disrepair in the aged lung. American Journal of Medical Sciences. 344 (1), 41-51 (2012).
- Moodley, Y., et al. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. American Journal of Pathology. 175 (1), 303-313 (2009).
- How, C. K., et al. Induced pluripotent stem cells mediate the release of interferon gamma-induced protein 10 and alleviate bleomycin-induced lung inflammation and fibrosis. Shock. 39 (3), 261-270 (2013).
- Lee, R. H., et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 111 (47), 16766-16771 (2014).

