Evaluation of the Productivity of Social Wasp Colonies (Vespinae) and an Introduction to the Traditional Japanese Vespula Wasp Hunting Technique
Summary
This methodological paper evaluates the productivity of a social wasp colony by examining the number of meconia per 100 cells of comb, to estimate the total number of adults the wasps produced. The associated video describes how to search for Vespula wasp nests, a method developed by amateur wasp chasers.
Abstract
For vespine wasps, colony productivity is typically estimated by counting the number of larval cells. This paper presents an improved method that enables researchers to estimate more accurately the number of adults produced, counting the number of meconia (the stools left in the cells by wasp larvae when pupating into adults, per 100 cells) in each comb. This method can be applied before or after colony collapse (i.e., in active or inactive nests). The paper also describes how to locate wild Vespula wasp colonies by "flagging" wasp baits and chasing the wasp collecting them, using a method traditionally performed by local people in central Japan (as illustrated in the associated video). The Vespula chasing method described has several advantages: it is easy to reinitiate the chase from a point where the forager flying back to the nest was lost, and it is easy to pinpoint the nest location as marked wasps often lose their flag at the nest entrance. These methods for estimating colony productivity and collecting nests can be valuable for researchers studying social wasps.
Introduction
Every species is thought to develop an optimal strategy for survival and reproduction among a vast array of possible strategies. In natural selection, individuals with traits that maximize an individual's reproductive success will leave more offspring (and genes) to the next generation. Therefore, the number of offspring produced by an individual can be used as an indicator of the individual's relative evolutionary fitness. In a given ecological context, the comparison of the number of offspring produced relative to alternative behavioral strategies can help researchers predict the best strategy for optimizing fitness1.
Social Hymenoptera (such as wasps, bees, and ants) have a system of three different castes, which are workers (sterile females), queens (gynes), and males1. Only new queens (gynes) and males count toward fitness in social Hymenoptera. Worker production does not directly contribute to fitness since the worker is infertile. On the other hand, a queen that can produce a higher colony productivity (such as a higher number of total cells or a heavier nest) is considered to have a higher fitness in social Hymenoptera, regardless of the number of actually produced new queens and males (see, e.g.,Tibbetts and Reeve2 and Mattila and Seeley3). In general, it is difficult to precisely count the number of offspring produced by a colony of social Hymenoptera. In fact, the queens of many social insects live for more than 1 year (e.g., leaf-cutter ant queens can live >20 years4 and honeybee queens may live for 8 years5). In addition, one queen may produce thousands of reproductive offspring over the course of several weeks or months, even in annual species of genera Vespa and Vespula6,7,8. Furthermore, the lifespan of workers is shorter than that of their mother queen, and workers often die away from their nests. Hence, even if one could accurately count all adults in a nest at any given point in time, such a count would not accurately depict the number of offspring produced. Therefore, the number of offspring produced has been roughly estimated from the size of the nest, the number of workers in the nest, or the weight of the nest at a given point in time3,9,10. The number of larval cells could result in an overestimation of the offspring production when some cells are empty. The same method could also result in a potential underestimation of the offspring production because combs of small cells that contain worker brood can produce two or three cohorts of larvae6,7,11.
The first aim of this work is to provide an improved method for estimating vespine wasp colony productivity in terms of the number of adults produced. Yamane and Yamane suggested that the best way to estimate the number of offspring produced by a colony is to count the meconia in the nest12. The meconia is the fecal pellet comprising larval cuticle, gut, and gut contents that a larva leaves in its cell when pupating (Figure 1A). The total number of meconia produced per comb is calculated by multiplying the total number of cells present by the average number of meconia per cell. There are often multiple layers of meconia in a cell, and each meconia indicates that an individual successfully pupated in that cell6,11 (Figure 1B). When estimating the mean number of meconia per cell, if the number of cells examined is small (a small sample size), the standard error (SE) increases, and as a result, the error for the total number of meconia per comb becomes higher than if the sample size was larger. The SE of the mean (SEM) is a measure of the dispersion of sample means around the population mean. Therefore, in this study, I focus on the SEM of the number of meconia per cell to estimate the population (the number of adults produced) from the sample mean (the average number of meconia per cell). This study attempts to determine how many samples are required to obtain an SE rate of less than 0.05 per cell. To do this, a numerical simulation is performed with real data on the number of meconia per comb, to determine the minimum sample size (for both worker and queen combs) needed to estimate this value accurately within the defined SE of 0.05.
Vespine wasp colonies live in concealed nests (underground or aerial) composed of multiple horizontal combs, built in series from top to bottom6,7,11. The average size of the cells increases from the first (top) to the last (bottom) comb. In the bottom combs, a sudden shift in the average cell size can be seen. These wider cells are built for the development of new queens. Hence, a more accurate estimate of colony productivity (i.e., the number of individuals produced) can be obtained when the total number of meconia in the worker cells (small cells) and queen cells (large cells) are considered. In order to estimate fitness at the colony level, researchers could estimate the number of queens produced and focus on the meconia in the queen cells alone. As for reproductive males, these are reared either in worker or queen cells, depending on the species. Thus, it may be difficult to estimate the male production of a colony, except in species where males have a third, unique cell size13 (e.g., Dolichovespula arenaria).
The second aim of this work is to present a useful technique for locating wild vespine wasp colonies in the field and transplanting them into laboratory nest boxes. Although some researchers obtain wasp nests from pest control calls (i.e., people reporting them as pests14,15), this method is not always possible or desirable. Researchers might need to collect nests in wild and inhabited areas where pest controllers do not operate, or to conduct their research by more flexibly obtaining nests at specific times. Interestingly, people living in the mountainous areas of central Japan traditionally collect and rear wasps (Vespula shidai, Vespula flaviceps, and Vespula vulgaris) for food. Therefore, collecting and artificial rearing techniques for these wasps are well developed in those areas17.
This paper also summarizes the methods employed to rear Vespula wasps. The experimental organism for this study was V. shidai, a social, ground-nesting wasp inhabiting western Asia and Japan. V. shidai possesses the largest colony size among all Japanese vespine wasps, with a total of 8,000 to 12,000 cells per nest, with a maximum of 33,400 cells14,18. Workers of V. shidai have an average wet weight of 67.62 ± 9.56 mg. Males are usually reared in worker cells; in contrast, new queens are reared in specially constructed, wider queen cells14.
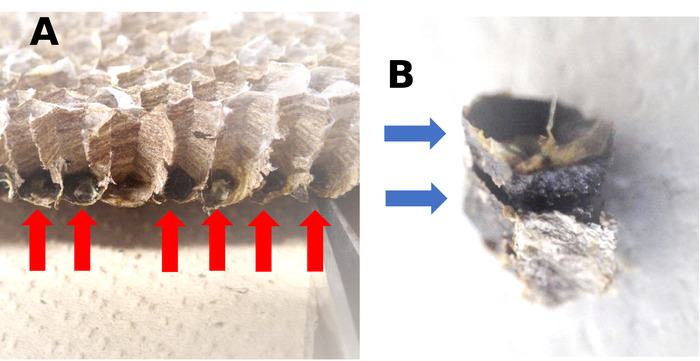
Figure 1: Meconium in a larval cell. (A) Cross section of a comb of Vespula shidai. Meconia is indicated by red arrows. (B) Two meconia are layered. Each blue arrow indicates one meconium. Please click here to view a larger version of this figure.
Protocol
1. Evaluation of Colony Productivity
- Estimation of the number of cells per comb
- Separate the combs one by one. Sweep away all adult wasps from the comb and pull out all larvae and pupae from the cells with tweezers.
- Measure the square measures of 10 randomly chosen cells per a comb, by using imaging software (e.g., Image J version 1.48, see http://imagej.nih.gov/ij/).
- Take a picture with the scale reference so that all the cells are pictured from right above.
- Based on the actual length of the scale, convert all measured lengths to pixels.
- Measure the areas of the 10 cells in pixels and convert them to the actual areas.
- Calculate the average area of worker and queen cells.
- Estimate the number of worker and queen cells by dividing the area of each comb by the average cell area per comb.
- Counting the number of meconia for the evaluation of colony productivity
- Count the number of meconia per 100 cells for each comb by carefully breaking the comb and examining meconia.
NOTE: This number of cells was determined here to be sufficient (the SE of the number of meconia per cell is within 0.05, see the representative results section). Meconia may have solidified into two or more layers in the cell (Figure 1). - Calculate the average number of meconia per cell for these 100 cells.
- Calculate the total number of meconia for each comb (i.e., the number of individuals produced, the colony productivity), extrapolated from the estimated number of cells and the average number of meconia per cell for that comb.
- Count the number of meconia per 100 cells for each comb by carefully breaking the comb and examining meconia.
2. Finding Vespula Nests
- Baiting
- Hang pieces of cuttlefish, freshwater fish, or chicken heart (approximately 10 g in total) on a tree branch at a height that can be easily reached by hand (Figure 2).
- Place these baits along a transect (e.g., along a road crossing a forest or along a river) at 50 to 100 stations, with at least 5 m between each station.

Figure 2: Providing wasps with a flagged meat bait. (A) Baiting wasps with meat attached to the tip of a stick. (B) The piece of meat is tied with a thread to a plastic flag. (C) The wasp holds on the meat which is tied to the flag. Such “flagged” baits will increase the visibility of the flying forager. The photos in panels B and C were taken by Fumihiro Sato. Please click here to view a larger version of this figure.
- Providing wasps with a “flagged” bait
- Flag construction and attachment
- Cut plastic (polyethylene) bags into strips of 3 – 5 mm in width and 15 cm in length by using a box cutter.
- Prepare 1.5 mm3 of chicken heart or cuttlefish on a bamboo skewer or thin branch (the diameter of the meat bait can be 1 – 2 mm, less than 15 mg for a V. shidai worker; Figure 2).
- Tie a thread to the flag (plastic strip, less than 10 mg) and then to meat bait, attaching it within 3 mm from the flag (this is called the “flagged” bait). Cut off the loose thread above the knot.
NOTE: Use extremely fine polyester thread normally used with sewing machines.
- Presentation of the meat bait to a wasp
NOTE: A nest is found most efficiently by following wasps that return to the bait repeatedly within 4 min of leaving. This is because wasps that take the bait and return quickly have a nest nearby.- Paint a unique mark on each thorax to identify the wasps individually when they are biting the baits (preferable with water-based paint pens, see Table of Materials).
- Orient the flag with the thread under the wasp while it bites the flagged bait when presenting the bait to the wasp (place the flag so that it and the thread pass under the wasp’s abdomen from below its thorax).
- Following a marked wasp
- Gather the baits from the surrounding area, so that the returning wasp is more likely to return to the same spot, before following a wasp.
NOTE: Following marked wasps is best accomplished with a group of two or more people. At least one person stays on at the transect, providing the foraging wasps with flagged baits, while the other(s) follow the marked wasp. When more than one wasp is attracted to the same bait, mark and follow only wasps that fly away in the same direction. - Follow a wasp with a flagged bait.
- When a followed wasp lands somewhere on the way to its nest, gently lift the wasp with a long stick (branch) or fishing rod and watch it until it resumes flight.
NOTE: Be gentle and do not strike the resting wasp because it will drop the bait and fly away. - When the wasp is shaping another meat ball before flying back to its nest again, readjust the flag, if necessary.
NOTE: Wasps will sometimes land and chew through the thread, removing the flag from the meat bait. If this happens frequently, make the flags shorter to increase forager flying ability. - When a wasp escapes detection while being followed, wait for the wasp to return to the bait station on the transect before resuming the chase. This time, while the wasp is biting the new bait, carry the bait stick (and wasp) to the point where it had last escaped detection.
NOTE: Foraging wasps do not let go of their baits easily, and do not sting if handled gently. Hence, the wasp with the flagged bait can be moved to the desired location by holding the flag, without the wasp escaping.
- Gather the baits from the surrounding area, so that the returning wasp is more likely to return to the same spot, before following a wasp.
- Flag construction and attachment
3. Transfer of the Nest
- Structure of the carrying box
- Construct nest boxes of various sizes, from 10 to 20 cm in length and width and from 10 to 20 cm in height, to accommodate nests of various sizes.
NOTE: Boxes of this size are big enough to accommodate young nests of V. shidai (collected in Central Japan between mid-July and mid-August). Make a carrying box according to the nest size of each species, for each growth stage. - Construct the bamboo grid and attach it to the inside of the box, about 2 cm above the bottom of the box, to facilitate the placement of the nest inside the carrying box.
- Cover the bottom of the carrying box with newspaper and paste it to a wooden, removable board (Figure 3).
NOTE: The newspaper will, later, allow the wasps to chew through it as they build additional combs below the carrying box when this is placed in a nest box (see section 3.2).
- Construct nest boxes of various sizes, from 10 to 20 cm in length and width and from 10 to 20 cm in height, to accommodate nests of various sizes.
- Excavation of the nest
- Before the exposure of the whole nest
NOTE: Wear protective clothing to avoid being stung by the wasps defending their nest.- Once the wasp nest is found, excavate the nest.
- Vigorously stamp on the ground around the nest for about 10 to 20 min so that workers leaving and returning to the nest remain inside to protect it, to collect as many workers as possible.
NOTE: If the wasps continue to stay outside the nest, it is better to capture them using an insect net. Although the stamping is useful for V. flaviceps, V. shidai, and V. vulgaris, other species’ workers from the nest may attack the individual performing the stamping. In the case, skip this step. - Shine a light directly into the nest entrance to determine the direction in which the nest entrance runs. Use a finger to confirm the orientation of the nest hole, while gently excavated soil from around the nest.
- After the exposure of the whole nest
- When the whole nest is exposed, spread a cloth and place the nest on top of it to prevent wasps from escaping into the ground under the nest.
- Place the excavated nest into a wooden (carrying) box for transportation to the lab (Figure 3); then, cover it with branches and newspaper. Leave the top of the nest uncovered while it is in the box.
- Place the carrying box on a cloth for 5 to 10 min, until the wasps become calm.
- Collect any wasps in the vicinity with an insect net and transport them to the laboratory with the nest.
NOTE: As an alternative collection procedure, anesthetize the nest occupants by fanning celluloid smoke or diethyl ether into the nest before excavating it.
- Before the exposure of the whole nest

Figure 3: Carrying box. (A) Box for carrying nests collected in the field. (B) A bamboo grid is placed at on the bottom of the box. The two boxes in the image on the right are upside down. Please click here to view a larger version of this figure.
4. Rearing Vespula
- Structure of the nest box
NOTE: The nest box is made of wood, with dimensions of 50 cm in length and width and 70 cm in height for rearing V. shidai (a mature nest is approximately 40 cm in diameter in the wild). Make a nest box according to the nest size of the species to be reared.- Provide the nest box with an entrance hole (usually placed in the upper part of the box) to allow wasps to leave the nest to forage.
- Fill about 1/3 of the nest box with soil like that occurring at the location where the nest was collected.
- Install a wire mesh (with a mesh size of 1.5 cm2) at the entrance of the nest box to prevent any intrusion by other wasps (predators, such as Vespa mandarinia and Vespa simillima).
- Place two wooden bars in the nest box that can bear the carrying box (Figure 4).

Figure 4: Laboratory setup. (A) Setting a carrying box into a nest box used for a long-term study. Before placing the carrying box in the nest box, the wood board at the bottom of the carrying box was removed, leaving only the newspaper to cover the bottom of the nest. (B) A series of nest boxes with food resources hanging from a wire line. Please click here to view a larger version of this figure.
- Transplantation of the carrying box into the nest box
- Keep the nest box in a dry place while rearing wasps in the collected nests (i.e., somewhere not exposed to rain).
- Remove the wooden board at the bottom of the carrying box and put it in the nest box for a long-term study (Figure 4).
NOTE: Often, wasps will have bitten holes in the newspaper covering the bottom of the carrying box, and so, there is a danger of being stung by wasps escaping through the holes. Therefore, wear protective clothing when transplanting the nest.
- Feeding the wasps
- Place various types of meat (squid, freshwater fish, chicken breast, or chicken heart) and a 1:3 solution of honey and water at approximately 3 m from the nest box.
- Provide enough food for the feeding requirements of 1 day. Replenish fresh food every day (Vespinae do not forage on old/rotting meat).
Representative Results
One goal of this study was to determine how many samples are required to obtain an SEM of the number of meconia per cell which is less than 0.05. In this study, a comb with an average cell size of <20 mm2 was defined as a worker comb, whereas larger combs were defined as queen combs. I counted the number of cells for queen combs and worker combs (in this study, counts were made of six queen combs and six worker combs from five V. shidai colonies). The actual number of cells per comb was estimated from these data via extrapolation (Table 1).
| ID | State | Collection date | Area (mm2) | Estimated number of cells (ENC) | Actual number of cells (ANC) | Actual number of meconium (ANM) | Mean number of meconium in a cell | ANM /ENC |
| WW-Kb01 | Alive | 18-Oct-16 | 27756.7 | 1599.9 | 1433 | 2430 | 1.70 | 1.52 |
| WW-Kb02 | Alive | 18-Oct-16 | 4098 | 381.9 | 347 | 494 | 1.42 | 1.29 |
| WW-Kb02 | Alive | 18-Oct-16 | 22439.3 | 1118.9 | 986 | 1317 | 1.34 | 1.18 |
| WR-Ksb | Collapse | 3-Nov-16 | 19094.9 | 1098.6 | 1,181 | 974 | 0.82 | 0.89 |
| WR-Ksc | Collapse | 27-Nov-16 | 38,933.40 | 2,198.70 | 2,455 | 4,321 | 1.76 | 1.96 |
| WR-Kb05 | Collapse | 29-Nov-16 | 10970 | 860 | 763 | 1315 | 1.72 | 1.53 |
| QW-Kb01 | Alive | 18-Oct-16 | 29186.2 | 1094.4 | 1095 | 759 | 0.69 | 0.69 |
| QW-Kb01 | Alive | 18-Oct-16 | 36920.5 | 1361.6 | 1341 | 1075 | 0.80 | 0.79 |
| QW-Kb02 | Alive | 18-Oct-16 | 37295.9 | 1047.2 | 1080 | 1068 | 0.99 | 1.02 |
| QR-Ksb | Collapse | 3-Nov-16 | 24811.2 | 1011.9 | 893 | 701 | 0.78 | 0.69 |
| QR-Ksc | Collapse | 27-Nov-16 | 33352.8 | 1384.5 | 1241 | 1069 | 0.86 | 0.77 |
| QR-Kb05 | Collapse | 29-Nov-16 | 25157.6 | 1071.4 | 922 | 572 | 0.62 | 1.97 |
| WW = a worker comb from a wild nest, WR = a worker comb from a rearing nest, QW = a queen comb from a wild nest, QR = a queen comb from a rearing nest. Alive = viable wasp larvae in cells, Collapse = no viable larvae in cells. | ||||||||
Table 1: The actual and estimated numbers of cells in six worker combs and six queen combs and the number of meconia per comb. WW = a worker comb from a wild nest, WR = a worker comb from a rearing nest, QW = a queen comb from a wild nest, QR = a queen comb from a rearing nest. Alive = viable wasp larvae in cells, Collapse = no viable larvae in cells.
An analysis of the relationship between the sample size and the SEM of the number of meconia per cell demonstrated that the sample size should be established using a bootstrap approach based on the number of meconia counted (from real data). Using real data, the mean and standard deviation (SD) of the number of meconia per cell were calculated, with the number of samples set at 1,000 for each sample size (the number of cells to be examined were 1 to 500; Figure 5). I did not allow for an iterative extraction from the data at sampling. The SEM for the number of meconia per cell was calculated for each sample size for each set of real data. Then, the sample size at which the SEM was less than 0.05 was examined. All calculations were made using software R.3.2.4.19 This analysis showed that the SEM was <0.05 when the sample size was 100 cells (for both worker and queen combs) (Figure 5). Therefore, the following results are based on examining the number of meconia per 100 cells per comb.
The actual and estimated numbers of cells in six worker combs and six queen combs and the number of meconia per comb are shown in Table 1. The estimates of the number of cells in the worker combs, based on comb area measurements, were both higher and lower than the true count. The mean number of meconia in the cells of worker combs, which represents the number of workers produced, ranged from 1.96 times more than the number of estimated larval cells to 0.89 times less than the estimated number of cells (Table 1). In the queen combs, the actual number of cells was often less than the estimated number of cells. The number of meconia in the queen combs, which can represent a component of fitness (i.e., a part of the reproductive success of the founding queen), was 0.53 to 1.02 times the estimated number of cells.
All cells and meconia were counted in six randomly selected worker combs and six randomly selected queen combs from the five nests (Table 1). The total number of cells counted in the worker combs was 7,165, whereas the number of meconia counted in the worker combs was 10,851. The average number of cells per comb was 1,194.2 ± 720.3 (average ± SD), whereas the average number of meconia in the worker combs was 1,808.5 ± 1,368.2. In the queen combs, the total number of all cells was 6,572, whereas the number of all meconia was 5,244. The average number of cells per comb in the queen combs was 1,095.3 ± 174.820, whereas the average number of meconia was 874.0 ± 223.8. Meconium layers in worker cells ranged from zero to three, whereas the queen cells had either one or no meconium layer.
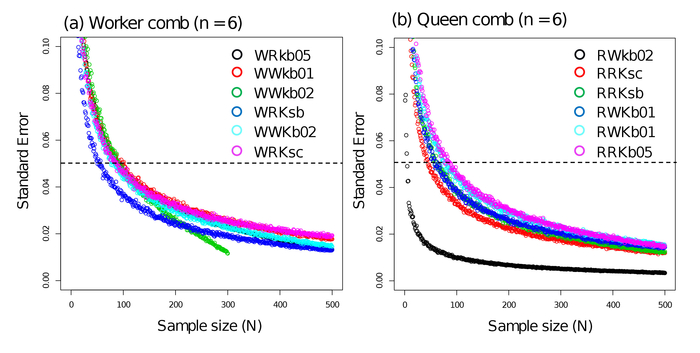
Figure 5: The relationship between the sample size and the standard error (SE) relative to the number of meconia counts. (a) Meconia per cell in worker combs. (b) Meconia per cell in queen combs. Each circle depicts an SE relative to the number of meconia per cell obtained via simulation with actual data. Color differences represent the data from each sampled nest. Simulating the SE for the number of meconia per cell in comb WWkb02 (worker comb) was accomplished with a sample size of 300 because that comb only had 347 cells. Please click here to view a larger version of this figure.
Discussion
The colony productivity of bees, ants, and wasps has been estimated previously by the number of workers and cells in nests or by the weight of the nests3,9,10. This study shows that the estimate of the number of meconia provides a better estimate of the overall number of individuals produced (i.e., a better indicator of colony productivity). In fact, it was found that, for both worker and queen combs, the number of meconia ranged from 0.53 to 1.96 times the number of larval cells in the comb. These findings quantify how inaccurate the determination of the number of workers and queens produced can be when it is based on the number of cells in a comb. Despite being more labor-intensive, estimating the number of meconia in a nest seems to guarantee a more precise evaluation of colony productivity. On the other hand, in this study, it was not evaluated how accurately the number of meconia represents the number of individuals produced.
This paper shows how many cells of a V. shidai nest should be examined to estimate colony productivity, based on the results of a bootstrap simulation approach using sample data on the number of meconia in the nest. Based on these results, it would be appropriate to investigate 100 cells per comb of both worker and queen cells. The method for counting meconia can also be applied to a nest after it has collapsed (i.e., is inactive), which can be advantageous for researchers: the reproductive period of vespine wasp colonies is quite long8 and studying a nest after it has collapsed means that the total number of adults produced over the entire reproductive period can be estimated. Such colonies are also easier to collect.
To collect nests of V. shidai, some researchers have followed either marked (e.g., coated with fluorescent powder) or unmarked wasps21. The nest location method presented here (feeding the wasps "flagged" meat) facilitates following wasps to their nests. This approach is also helpful if a tracked wasp is lost because the same wasp will eventually return to the bait along the transect. Provide new flagged bait to this wasp and carry it to the point where it was last lost, thus allowing chasers to resume the chase from that point onward (closer to the nest). Some of the flags brought to the nest are dislodged at the nest entrance, which also facilitates finding ground nests. However, this method is not suitable for rainy days because markers tend to stick to branches and leaves when they get wet. Although chasing flagged wasps is useful for V. shidai, V. flaviceps, and V. vulgaris in Japan, this method could not be applied to Vespula rufa because these wasps do not come to the bait and do not grab flagged bait. The nest location method can probably not be used for some Vespula wasps.
More sustainable diets are needed by an ever-increasing global population. In addition, the demand for edible insects increases daily. Many edible insects, which are being consumed locally and traditionally throughout the world, have been identified by the Food and Agriculture Organization of the United Nations21 as a promising alternative protein source for overcoming food insecurity worldwide. Larvae and pupae of Vespula have traditionally been used as food in mountainous areas of Japan16, and so, they could be used to provide a source of protein elsewhere in the world. The set of protocols developed in this study is likely applicable to locating nests of other yellowjacket species. Therefore, the protocols outlined in this paper will be useful for collecting yellowjackets as an edible resource and studying wasp behavior.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The author would like to thank Katsuyuki Takahashi, Hiroo Kobayashi, Fumihiro Sato, Daikichi Ogiso, Toshihiro Hayakawa, and Hisaki Imai for teaching him the traditional wasp hunt method. The author would like to offer special thanks to Kevin J. Loope and Davide Santoro for carefully proofreading the manuscript. The author is grateful to Masato Abe, Yasukazu Okada, Yuichiro Kobayashi, Masakazu Shimada, and Koji Tsuchida for their discussion. The author wants to thank Yuya Shimizu and Haruna Fujioka for their technical assistance with evaluating colony productivity. The author would like to thank Tsukechi black bee club for supporting the video shooting. The author wishes to thank three anonymous reviewers for their comments on an early version of this paper. This study was supported in part by Takeda Science Foundation, Fujiwara Natural History Foundation, Funding of the Nagano Society for The Promotion of Science, Shimonaka Memories Foundation, Takara Harmonist Fund, and the Dream Project by Come on UP, Ltd.
Materials
| cuttlefish | Any | fresh/ as a bait | |
| dace | Any | fresh/ as a bait | |
| chichken heart | Any | fresh/ as a bait | |
| plastic bag (polyethylene) | Any | as a flag | |
| bamboo skewer | Any | ||
| industrial sewing thread | FUJIX Ltd. | King polyester, No.100 | |
| paint marker pen | Mitsubishi pencil | UNI, POSCA, PC5M | |
| fishing rod | ANY | ||
| carrying box | made of wood | ||
| nest box | made of wood |
References
- Davies, N. B., Krebs, J. R., West, S. A. . An introduction to Behavioural Ecology. , (2012).
- Tibbetts, E. A., Reeve, H. K. Benefits of foundress associations in the paper wasp Polistes dominulus: increased productivity and survival, but no assurance of fitness returns. Behavioural Ecology. 14, 510-514 (2003).
- Mattila, H. R., Seeley, T. D. Genetic Diversity in Honey Bee colonies Enhances Productivity and Fitness. Science. 317, 362 (2007).
- Weber, N. A. Gardening Ants, the Attines. American Philosophical Society. , (1972).
- Baer, B., Schmid-Hempel, P. Sperm influences female hibernation success, survival and fitness in the bumble-bee Bombus terrestris. Proceedings: Biological Science. 272 (1560), 319-323 (2005).
- Spradbery, J. P. . Wasps. An Account of the Biology and Natural History of Social and Solitary Wasps, with Particular Reference to Those of the British Isles. , (1973).
- Matsuura, M., Yamane, S. . Comparative Ethology of the Vespine Wasps. , (1984).
- Greene, A. Production schedules of vespine wasps: an empirical test of the bang-bang optimization model. Journal of Kansas Entomological Society. 57 (4), 545-568 (1984).
- Cole, B. J. Multiple mating and the evolution of social behavior in the Hymenoptera. Behavior Ecology Sociobiology. 12, 191-201 (1983).
- Goodisman, M. A. D., Kovacs, J. L., Hoffman, E. A. The significance of multiple mating in the social wasps Vespula maculifrons. Evolution. 61 (9), 2260-2267 (2007).
- Greene, A., Ross, K. G., Matthews, R. W. Dolichovespula and Vespula. The Social Biology of Wasps. , 263-305 (1991).
- Yamane, S., Yamane, S. Investigating methods of dead vespine nests (Hymenoptera, Vespidae) (Methods of taxonomic and bio-sociological studies on social wasps. II). Teaching Materials for Biology. 12, 18-39 (1975).
- Loope, K. J. Matricide and queen sex allocation in a yellowjacket wasp. The Science of Nature. 103 (57), 1-11 (2016).
- Matsuura, M. . Social Wasps of Japan in Color. , (1995).
- Foster, K. R., Ratnieks, F. L. W., Gyllenstrand, N., Thoren, P. A. Colony kin structure and male production in Dolichovespula wasps. Molecular Ecology. 10 (4), 1003-1010 (2001).
- Loope, K. J., Chien, C., Juhl, M. Colony size is linked to paternity frequency and paternity skew in yellowjacket wasps and hornets. BMC Evolutionary Biology. 14 (1), 1-12 (2014).
- Nonaka, K. Cultural and commercial roles of edible wasps in Japan. Forest Insects as Food: Humans Bite Back. Proceedings of a workshop on Asia-Pacific resources and their potential for development. , 123-130 (2010).
- Yamane, S., Funakoshi, K. The unique ecology of Vespula shidai amamiana and the origin of distribution. Ecological Society of Japan. Biodiversity of the Nansei Islands, its formation and conservation. , (2015).
- . R: The R Project for Statistical Computing Available from: https://www.R-project.org/ (2018)
- Saga, T., Kanai, M., Shimada, M., Okada, Y. Mutual intra- and interspecific social parasitism between parapatric sister species of Vespula wasps. Insectes Sociaux. 64 (1), 95-101 (2017).
- Van Huis, A., et al. . Edible insects: future prospects for food and feed security. , (2013).

