Separation of Uranium and Thorium for 230Th-U Dating of Submarine Hydrothermal Sulfides
Summary
The protocol describes a method to purify and separate the U and Th nuclide in submarine hydrothermal sulfide sample with Fe co-precipitation and extraction chromatography for 230Th-U disequilibrium dating.
Abstract
The age of a submarine hydrothermal sulfide is a significant index for estimating the size of hydrothermal ore deposits. Uranium and thorium isotopes in the samples can be separated for 230Th-U dating. This article presents a method to purify and separate U and Th isotopes in submarine hydrothermal sulfide samples. Following this technique, the separated U and Th fractions can meet measuring requirements by multi-collector inductively coupled plasma mass spectrometry (MC-ICPMS). The age of the hydrothermal sulfide sample can be calculated by measuring the present-day activity ratios of 230Th/238U and 234U/238U. A super clean room is necessary for this experiment. Cleaned regents and supplies are used to reduce the contamination during the sample processes. Balance, hotplate, and centrifuge are also used. The sulfide sample is powdered for analysis and less than 0.2 g sample is used. Briefly, the sample is weighed, dissolved, added to 229Th-233U-236U double spike solution, Fe co-precipitated, and separated on an anion-exchange resin extraction column. Approximately 50 ng U is consumed for 230Th-U dating of sulfides sample by MC-ICPMS.
Introduction
Submarine hydrothermal sulfides have been a steady source of metals like iron, copper, zinc and lead. They are also seen as economically viable resources of silver and gold. The location and size of the deposits are a record of the history of hydrothermal venting on the seafloor. Dating of a hydrothermal sulfide can provide important information regarding the formation and alteration mechanism of the sulfide ore deposit, seafloor hydrothermal activity history, and growth rate of large sulfide deposits1,2,3. 238U-234U-230Th disequilibrium dating is an effective isotopic method of age estimation for hydrothermal sulfides4,5,6,7,8,9,10,11,12, where the purification and separation of U and Th isotopes is necessary. This text describes a protocol for U and Th isotopes separation and 230Th-U dating of sulfides sample by MC-ICPMS.
Geological materials which contain U and Th remain undisturbed for several million years, and a state of secular equilibrium between all the nuclides in the radioactive series is established. However, a combination of chemical solubility and nuclear recoil factors often create disequilibrium, in which the members of the decay series are separated from each other through processes such as deposition, transport and weathering. For example, when a sulfide deposit is formed, the state of 238U, 234U and 230Th is of disequilibrium, and the long-lived 238U can decay gradually towards short-lived 234U and 230Th subsequently. Assuming (i) the system remains closed with respect to U and Th isotopes, and (ii) initial amount of 230Th and 232Th incorporated into sulfide samples is zero, it is possible to determine the time of deposition by measuring the present-day activity ratios of 230Th/238U and 234U/238U. However, the initial amount of Th is not zero in the sample, and we assume the initial 230Th/232Th atomic ratio is 4.4 ± 2.2 x 10-6. The applicable dating range of this method is approximately ~10-6 x 105 years13,14. However, the large difference between the abundance of uranium and thorium makes measurement challenging. Hence, it is very important to establish a chemical procedure for U-Th dating by MC-ICPMS.
In the past 30 years, most studies focused more measurements of carbonate materials14,15,16,17 and less on sulfide deposits11,12,18,19. Alpha-particle counting methods have traditionally been used for the study of 230Th/238U disequilibrium of submarine hydrothermal sulfides1. However, analytical uncertainty of 5-17% is a limiting factor that affects the precision of age determination of sulfides1,8,9. These techniques generally suffer from the use of relatively large columns and reagent volumes and the need for multiple column passes for purification and separation U-Th from a sample. Recent developments in MC-ICPMS have greatly improved the precision of U-Th isotopic measurements (<5‰ for ages)14 and have significantly reduced the sample size (<0.2 g) required for analysis. In these works, many chemical separation procedures have been developed, and have achieved excellent chemical yields with low chemical background12,13.
Here we present a chemical-based protocol to obtain samples that are sufficiently clean for MC-ICPMS analysis. It is suitable for the dating of hydrothermal sulfide samples of age <6 x 105 years14. With this technique, the separated U and Th isotopic fractions can meet measuring requirements by MC-ICPMS. The age of the hydrothermal sulfide sample can be calculated from the extent of disequilibria between 230Th and 234U and between 234U and 238U by using the described activity decay equation.
Protocol
1. Preparing the sample, reagents, and containers
- Clean the fume hood, hotplate and the balance room bench for the chemical experiment with sprayed alcohol or ultrapure water.
- Prepare sub-boiled acids (2 M HCl, 8 M HCl, 7 M HNO3, and 14 M HNO3), clean beakers and any apparatus before sample processed.
NOTE: Sulfide samples presented in this study were collected from newly discovered hydrothermal zones in the South Atlantic. Approximately 60 mg of powdered sample was used in this process. Sample was collected into glass vials and put in the sample storage cabinet.
2. Weigh the samples
- Prepare cleaned 30 mL perfluoroalkoxy (PFA) beakers. Label twice outside the beaker (to prevent erasure).
- Weigh the blank beakers.
NOTE: The balance used is accurate to ±0.0001 g provided that all the vessels have had their static electricity completely removed. - Read the weight and record it.
- Pour the sample into the beaker. Cover with a lid and weigh the samples.
NOTE: Sample weight depends on the 230Th content. 230Th level varies with the U concentration and age of the sample. In general, a total of 100 ng of total U is sufficient for the sample. - Add some (~1 mL) ultrapure water using a bottle, rinse the inner wall and shake the beaker carefully.
NOTE: Add enough ultrapure water cover all the samples.
3. Dissolve and spike the sample
- Place the sample-containing beaker in the fume hood.
- Open the beaker lid. Add 3 mL of HNO3 (14 M) or aqua regia into the sample using a pipette.
- Place the beaker on the hotplate, set the hotplate temperature to 170 °C and dissolve the sample completely.
NOTE: If there are still insoluble substances in the solution, add 12 M HCl, 22.6 M HF and 10.6 M HClO4, and use a pressurized closed tank to ensure complete dissolution of samples. - Leave the solution to cool for at least 30 min. Add 0.1–0.3 g 229Th-233U-236U spike solution of known activity into the solution.
NOTE: Generally, the optimal ratio of 235U/233U is ~10–20:1 in the mixed solution. - Place the solution onto the hotplate, set the temperature to 170 °C and leave it on the hotplate until it dries.
NOTE: Evaporation must be done slowly when the sample approaches dryness. - Dissolve the sample in 2 drops of HNO3 (0.04 mL, 14 M), and dry it on the hotplate at 170 °C again.
4. Ferric hydroxide co-precipitation for U-Th
- Prepare cleaned 15 mL centrifuge tubes, label and place them in the tube stand.
NOTE: Add approximately 10 mg of Fe(III) (FeCl3 in 12 M HCl) into the centrifugal tube carefully if samples contain almost no Fe. - Add several drops (0.1 mL) of 2 M HCl into the beaker. Shake the beaker gently and dissolve the sample completely.
- Transfer each sample into a centrifuge tube.
- Add several drops of ammonia (~0.1 mL) until the acid is neutralized; when pH is 7–8, a reddish-brown precipitate appears. U and Th isotopes are precipitated by the Fe(OH)3.
NOTE: The clear solution contains unwanted ions such as metal-elements, Mg2+, NO3– and NH4OH. - Cap the centrifuge tubes. Centrifuge at 2,340 x g for 7 min. Discard the supernatant
- Add some ultrapure water to wash the precipitate. Centrifuge as above and repeat this step twice more.
- Dissolve the precipitate with 1.5 mL of 7 M HNO3. Transfer it into the corresponding beaker.
- Add 1 drop of HClO4 (to remove organic matter), and dry it on the hotplate at 170 °C for about 30 min.
5. Preparation of anion exchange column
- Prepare small polytetrafluoroethylene (PTFE) columns (~2.5 mL column size) as shown in Figure 1; insert the frit into each column slowly at the bottom on the bench.
- Pipet cleaned anion-exchange resins into the columns. Put the columns on the holder.
- Fill the whole column with ultrapure water. Add 1 drop of 14 M HNO3.
NOTE: This step is performed in order mainly to remove the trace elements in the column. - Add 2 column volumes (CV) of 7 M HNO3 to remove the trace elements. Then repeat this step.
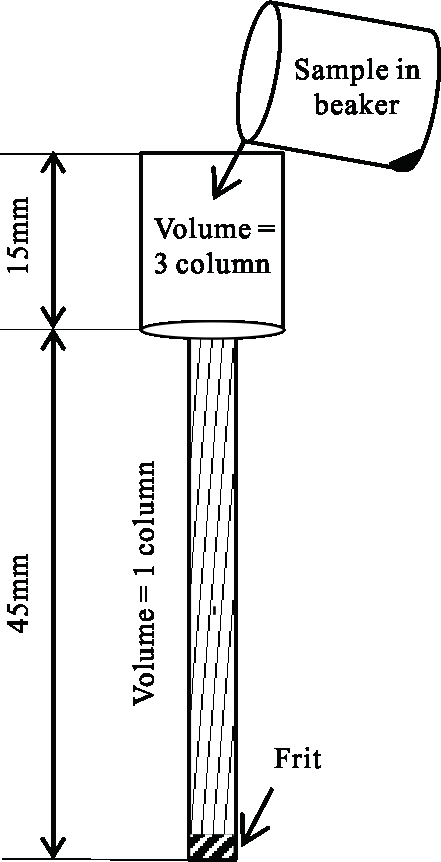
Figure 1: Ion-exchange column filling with anionic exchange resin. Please click here to view a larger version of this figure.
6. Purification and separation of U and Th fractions
- Dissolve the sample in 0.5 mL of 7 M HNO3. Load it onto the column carefully.
- Let it drip across the column into the waste beaker.
- Add 2 CV and 1 CV of 7 M HNO3 successively into the column. Iron and other metal-elements in the sample are removed while U and Th are retained by the resin in this step.
- Add 2 CV and 1 CV of 8 M HCl into the column successively to elute thorium fraction. Collect the thorium fraction using a 7 mL capacity cleaned PFA beaker. Add 1 drop of HClO4 into the beaker and dry the fraction on a hotplate at 170 °C for about 30 min.
- Elute uranium fraction from the resin with 2 CV of 0.1 M HNO3 twice. Collect the eluate in the cleaned PFA beaker. Add 1 drop of HClO4 and dry it on the hotplate at 170 °C for about 30 min.
- Prepare and label 2 mL capacity vials.
- Dissolve each sample in 1 drop HNO3 and dry it on the hotplate at 170 °C for less than 5 min until 0.5 drop is left. Transfer them along with 0.2 mL of 2% HNO3 + 0.1% HF into the corresponding vials for instrument measurement.
Figure 2: Uranium and thorium fractions of the submarine hydrothermal sulfides. Please click here to view a larger version of this figure.
7. MC-ICPMS measurement
- Measure the U and Th fractions collected through the above chemical purification process using a high-resolution MC-ICPMS instrument.
NOTE: U and Th isotopic ratios can be obtained by using the instrument by applying secondary electron multiplier (SEM)21 technique. The instrument parameters13 are listed in Table 1. Thorium age was calculated using the following equation:
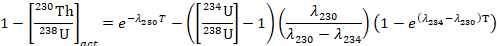
Initial ratio of 234U to 238U was measured as follows:
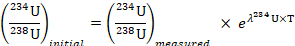
| Instrument | Parameter | Value |
| MC-ICPMS | RF power | 1325 W |
| Cool gas | 16.00 L min-1 | |
| Auxiliary gas | 1.78 L min-1 | |
| Sample gas | 1.00 L min-1 | |
| Low resolution | 300~400 | |
| CETAC Aridus II | Sample injection rate | 50~60 μL min-1 |
| Ar Sweep Gas | 2~5 L min-1 | |
| Nitrogen Gas | 2~10 mL min-1 | |
| Spray Chamber Temperature | 110 °C | |
| Membrane Oven Temperature | 160 °C |
Table 1: Instrument parameters for measuring U-Th isotopes by MC-ICPMS (using the instrument listed in the Table of Materials).
Representative Results
Using this procure, a submarine hydrothermal sulfide sample can be completely dissolved. Following this protocol, the Th fraction was eluted from the hydrothermal sulfide sample using 8 M HCl. Meanwhile, the U fraction of the hydrothermal sulfide sample was eluted with 0.1 M HNO3. U and Th fractions were dissolved in the 2% HNO3 (+0.1% HF) solution (see Figure 2) and stored in 2 mL capacity vials. The mixture was then analyzed by MC-ICPMS.
With the MC-ICPMS instrument, U and Th isotopes ratio and the age of submarine hydrothermal sulfide is determined precisely. The ages were calculated by an iterative method13. The test results are listed in Table 2. U content ranged from 178.0 to 5,118.2 ng·g-1, and Th content ranged from 603 to 7,212 pg·g-1. Five samples had ages of 567 ± 52, 1,585 ± 27, 3,345 ± 132, 14,211 ± 727 and 21,936 ± 91 years B.P. (B.P. stands for “before year 2000 A.D.”). Sample consumption was about 60 mg except S32 wherein only 17 mg sample was consumed.
| Sample | Sample Mass | 238U | 232Th | 230Th/232Thb | 234U/238Ub | 230Th/238Ub | 230Th Age(yr)c | 230Th Age (yr BP)d, e | (234U/238U)initialf | ||||||||
| No. | (mg)a | (ng g-1) | (pg g-1) | (uncorrected) | (corrected) | ||||||||||||
| S12 | 58 | 182.8 | ±0.2 | 7212 | ±144 | 11.7 | ±0.3 | 1.156 | ±0.002 | 0.1511 | ±0.0018 | 15221 | ±193 | 14211 | ±727 | 1.163 | ±0.002 |
| S15 | 57 | 569.3 | ±0.7 | 1200 | ±24 | 310.3 | ±6.3 | 1.166 | ±0.002 | 0.2140 | ±0.0007 | 22006 | ±84 | 21936 | ±91 | 1.177 | ±0.002 |
| S32 | 17 | 5118.2 | ±10.4 | 5173 | ±104 | 51.9 | ±1.2 | 1.157 | ±0.003 | 0.0172 | ±0.0002 | 1628 | ±20 | 1585 | ±27 | 1.158 | ±0.002 |
| Y3 | 55 | 178.0 | ±0.2 | 865 | ±17 | 23.0 | ±0.8 | 1.162 | ±0.002 | 0.0366 | ±0.0010 | 3484 | ±100 | 3345 | ±132 | 1.164 | ±0.002 |
| Y4 | 59 | 347.1 | ±0.4 | 603 | ±12 | 11.7 | ±0.8 | 1.159 | ±0.002 | 0.0067 | ±0.0004 | 629 | ±42 | 567 | ±52 | 1.159 | ±0.002 |
| a Sample mass for separation of uranium and thorium nuclide and U and Th analysis. | |||||||||||||||||
| b All ratios are radioactivity ratio, which calculated based on the decay constants λ238=1.55125 ×10-10 a-1 as described by Jaffey et al.(1971)20, λ234=2.82206 (±0.00302)×10-6 a-1 as described by Cheng et al.(2013)15, and 9.1705(±0.0138)×10-6 a-1 as described by Cheng et al.(2013)15. | |||||||||||||||||
| c Calculated 230Th age following the equation | 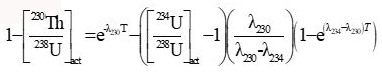 |
||||||||||||||||
| d Corrected 230Th ages assume the initial 230Th/232Th atomic ratio of 4.4 ±2.2 x10-6. Those are the values for a material at secular equilibrium, with the bulk earth 232Th/238U value of 3.8. The errors are arbitrarily assumed to be 50%15. | |||||||||||||||||
| e B.P. stands for “Before year 2000 A.D.”. | |||||||||||||||||
| f | 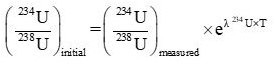 |
||||||||||||||||
Table 2. 230Th dating results for submarine hydrothermal sulfides. The error shown is 2s error.
aSample mass for separation of uranium and thorium nuclide and U and Th analysis.
bAll ratios are radioactivity ratios, which are calculated based on the decay constants λ238 = 1.55125 x 10-10 a-1 as described by Jaffey et al.20, λ234 = 2.82206 (±0.00302) x 10-6 a-1 as described by Cheng et al.15, and 9.1705 (±0.0138) x 10-6 a-1 as described by Cheng et al.15.
cCalculated 230Th age following the equation in section 7.
dCorrected 230Th ages assuming the initial 230Th/232Th atomic ratio to be 4.4 ± 2.2 x 10-6. These are the values for a material at secular equilibrium, with the bulk earth 232Th/238U value of 3.8. The errors are arbitrarily assumed to be 50%.
eB.P. stands for “Before year 2000 A.D.”.
fUsing the equation in section 7.
Discussion
Some critical steps must be followed to ensure success of this protocol. Ensure that all operations are carried out in clean chemistry room under fume hood with clean air circulation. Purify all regents in this process in advance and clean the apparatus before use. Dissolve the samples completely in the process of making the 7 M HNO3 solution which is then loaded onto the 7 M HNO3-conditioned resins. If there is any insoluble substance in the sample, it will be redissolved after drying. Additional important steps are suggested: (i) avoid the cross contamination from the adjacent samples during the sample processing; (ii) for each elution step allow the liquid to drain completely before the next step; and (iii) complete the process from the conditioning of the columns to collecting Th and U fractions within 2 h, otherwise the strong acid tends to break down the resin.
The major limitation of this technique is related to the 238U and 232Th concentration of the sample. It is best to choose samples with U > 50 ppb and Th < 10 ppb. The AG 1-X8 resin used can be replaced by UTEVA resin in the process.
With this method, five submarine hydrothermal sulfides samples from the South Atlantic were measured. Ages were 567 ± 52 to 21,936 ± 91 year B.P., indicating that this region has been experiencing hydrothermal activity events from 21,936 ± 91 years B.P.
U-Th purification and separation refers to isotopic methods of age estimation based on the measurement of uranium (238U and 235U), thorium (232Th), and certain members of the intermediate daughter nuclides in the three naturally occurring radioactive decay series for hydrothermal sulfide sample. It is also useful to determine the U and Th concentration of deep-sea sediments19. The technique can be applied to the dating of carbonate and phosphate, and to environmental tracer studies, assisting in building the age framework for the formation of minerals.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was financially supported by Experimental Technology Innovation Foundation of Institute of Geology and Geophysics, Chinese Academy of Sciences (No. 11890940), and China Ocean Mineral Resources R & D Association Project (No. DY135-S2-2-07).
Materials
| AG 1-X8 anion-exchange resin | BIO-RAD | 140-1441 | Separating rare elements |
| Ammonia solution | Kanto Chemical CO., INC. | 1336-21-6 | Reagent |
| Glass vials | BOTEX | None | Sample collection |
| Hydrochloric acid | Sinopharem chemical reagent Co. Ltd | 7647-01-0 | Reagent |
| Hydrofluoric acid | EMD Millipore CO. | 7664-39-5 | Reagent |
| Neptune Plus | Thermo Fisher Scientific CO. | None | Apparatus |
| Nitric acid | Sinopharem chemical reagent Co. Ltd | 7697-37-2 | Reagent |
| Perchloric acid | Kanto Chemical CO., INC. | 32059-1B | Reagent |
| Ultrapure water | Merck Millipore | None | Producted by Mill-Q Advantage systerm |
| Wipe paper | Kimberley-Clark | 0123-12 | Wipe and clean |
| 2 ml vial | Nelgene | 5000-0020 | Sample collection |
| 229Th-233U-236U spike | None | None | Reagent |
| 7 ml PFA beaker | Savillex | 200-007-20 | Sample treatment |
| 10 ml centrifuge | Nelgene | 3110-1000 | Sample treatment |
| 30 ml PFA beaker | Savillex | 200-007-20 | Sample treatment |
References
- Lalou, C., Brichet, E., Hekinian, R. Age dating of sulfide deposits from axial and off-axial structures on the East Pacific Rise near 12°500N. Earth and Planetary Science Letters. 75 (1), 59-71 (1985).
- Lalou, C., Brichet, E. On the isotopic chronology of submarine hydrothermal deposits. Chemical Geology. 65 (3-4), 197-207 (1987).
- Lalou, C., Reyss, J. L., Brichet, E. Actinide-series disequilibrium as a tool to establish the chronology of deep-sea hydrothermal activity. Geochimica et Cosmochimica Acta. 57 (6), 1221-1231 (1993).
- Lalou, C., et al. New age data for Mid-Atlantic Ridge hydrothermal sites: TAG and Snakepit chronology revisited. Journal of Geophysical Research. 98, 9705-9713 (1993).
- Lalou, C., Reyss, J. L., Brichet, E., Rona, P. A., Thompson, G. Hydrothermal activity on a 105-year scale at a slow-spreading ridge, TAG hydrothermal field, Mid-Atlantic Ridge 26° N. Journal of Geophysical Research. 100 (B9), 17855-17862 (1995).
- Kadko, D. Radio isotopic studies of submarine hydrothermal vents. Reviews of Geophysics. 34 (3), 349-366 (1996).
- Lalou, C., Mu ̈nch, U., Halbach, P., Reyss, J. Radiochronological investigation of hydrothermal deposits from the MESO zone, Central Indian Ridge. Marine Geology. 149 (149), 243-254 (1998).
- Yejian, W., et al. Hydrothermal Activity Events at Kairei Field, Central Indian Ridge 25°S. Resource Geology. 62 (2), 208-214 (2012).
- Yejian, W., et al. Mineralogy and geochemistry of hydrothermal precipitates from Kairei hydrothermal field, Central Indian Ridge. Marine Geology. 354 (3), 69-80 (2014).
- Jun-ichiro, I., et al. Dating of Hydrothermal Mineralization in Active Hydrothermal Fields in the Southern Mariana Trough. Subseafloor Biosphere Linked to Hydrothermal Systems. , 289-300 (2015).
- Takamasa, A., et al. U-Th radioactive disequilibrium and ESR dating of a barite-containing sulfide crust from South Mariana Trough. Quaternary Geochronology. 15 (1), 38-46 (2013).
- Weifang, Y., et al. 230Th/238U dating of hydrothermal sulfides from Duanqiao hydrothermal field, Southwest Indian Ridge. Marine Geophysical Research. 38 (1-2), 71-83 (2017).
- Lisheng, W., Zhibang, M., Hai, C., Wuhui, D., Jule, X. Determination of 230Th age of Uranium-series standard samples by multiple collector inductively coupled plasma mass spectromerty. Journal of China Mass Spectrometry Society. 37 (3), 262-272 (2016).
- Wang, L., et al. U concentration and 234U/238U of seawater from the Okinawa Trough and Indian Ocean using MC-ICPMS with SEM protocols. Marine Chemistry. 196, 71-80 (2017).
- Hai, C., et al. Improvements in 230Th dating, 230Th and 234U half-life values, and U-Th isotopic measurements by multi-collector inductively coupled plasma mass spectrometry. Earth and Planetary Science Letters. , 82-91 (2013).
- Edwards, R. L., Chen, J. H., Ku, T. -. L., Wasserburg, G. J. Precise timing of the last interglacial period from mass spectrometric analysis of 230Th in corals. Science. 236 (4808), 1537-1553 (1987).
- Edwards, R. L., Taylor, F. W., Wasserburg, G. J. Dating earthquakes with high precision thorium-230 ages of very young corals [J]. Earth and Planetary Science Letters. 90 (4), 371-381 (1988).
- Hai, C., Jess, A., Edwards, R. L., Boyle, E. A. U-Th dating of deep-sea corals. Geochimica et Cosmochimica Acta. 64 (14), 2401-2416 (2000).
- Ishibashi, J., et al. Dating of Hydrothermal Mineralization in Active Hydrothermal Fields in the Southern Mariana Trough. Subseafloor Biosphere Linked to Hydrothermal Systems. , 289-300 (2015).
- Jaffey, A. H., Flynn, K. F., Glendenin, L. E., Bentley, W. C., Essling, A. M. Precision measurement of half-lives and specific activities of 235U and 238U. Physical Review C. 4, 1889-1906 (1971).
- Richter, S., Goldberg, S. A., Mason, P. B., Traina, A. J., Schwieters, J. B. Linearity tests for secondary electron multipliers used in isotope ratio mass spectrometry. International Journal of Mass Spectrometry. 206 (1-2), 105-127 (2001).

